The Implication of Androgens in the Presence of Protein Kinase C to Repair Alzheimer’s Disease-Induced Cognitive Dysfunction
- PMID: 31677609
- PMCID: PMC6984714
- DOI: 10.29252/ibj.24.2.64
The Implication of Androgens in the Presence of Protein Kinase C to Repair Alzheimer’s Disease-Induced Cognitive Dysfunction
Abstract
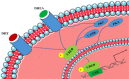
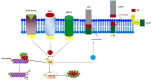
Aging, as a major risk factor of memory deficiency, affects neural signaling pathways in hippocampus. In particular, age-dependent androgens deficiency causes cognitive impairments. Several enzymes like protein kinase C (PKC) are involved in memory deficiency. Indeed, PKC regulatory process mediates α-secretase activation to cleave APP in β-amyloid cascade and tau proteins phosphorylation mechanism. Androgens and cortisol regulate PKC signaling pathways, affecting the modulation of receptor for activated C kinase 1. Mitogen-activated protein kinase/ERK signaling pathway depends on CREB activity in hippocampal neurons and is involved in regulatory processes via PKC and androgens. Therefore, testosterone and PKC contribute in the neuronal apoptosis. The present review summarizes the current status of androgens, PKC, and their influence on cognitive learning. Inconsistencies in experimental investigations related to this fundamental correlation are also discussed, with emphasis on the mentioned contributors as the probable potent candidates for learning and memory improvement.
Keywords: Androgens; Cognition; Hippocampus; Protein kinase C; Spatial memory.
Conflict of interest statement
None declared.
Figures
References
-
- Hojo Y, Hattori TA, Enami T, Furukawa A, Suzuki K, Ishii HT, Mukai H, Morrison JH, Janssen WG, Kominami S, Harada N, Kimoto T, Kawato S. Adult male rat hippocampus synthesizes estradiol from pregnenolone by cytochromes P45017α and P450 aromatase localized in neurons. Proceedings of the National academy of sciences of the United Stated of America. 2004;101(3):865–870. - PMC - PubMed
-
- Kimoto T, Tsurugizawa T, Ohta Y, Makino J, Tamura H, Hojo Y, Takata N, Kawato S. Neurosteroid synthesis by cytochrome p450-containing systems localized in the rat brain hippocampal neurons: N-methyl-D-aspartate and calcium-dependent synthesis. Endocrinology. 2001;142(8):3578–3589. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
