Thalamic degeneration in MPTP-treated Parkinsonian monkeys: impact upon glutamatergic innervation of striatal cholinergic interneurons
- PMID: 31679085
- PMCID: PMC6878768
- DOI: 10.1007/s00429-019-01967-w
Thalamic degeneration in MPTP-treated Parkinsonian monkeys: impact upon glutamatergic innervation of striatal cholinergic interneurons
Abstract
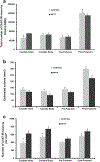
In both Parkinson's disease (PD) patients and MPTP-treated non-human primates, there is a profound neuronal degeneration of the intralaminar centromedian/parafascicular (CM/Pf) thalamic complex. Although this thalamic pathology has long been established in PD (and other neurodegenerative disorders), the impact of CM/Pf cell loss on the integrity of the thalamo-striatal glutamatergic system and its regulatory functions upon striatal neurons remain unknown. In the striatum, cholinergic interneurons (ChIs) are important constituents of the striatal microcircuitry and represent one of the main targets of CM/Pf-striatal projections. Using light and electron microscopy approaches, we have analyzed the potential impact of CM/Pf neuronal loss on the anatomy of the synaptic connections between thalamic terminals (vGluT2-positive) and ChIs neurons in the striatum of parkinsonian monkeys treated chronically with MPTP. The following conclusions can be drawn from our observations: (1) as reported in PD patients, and in our previous monkey study, CM/Pf neurons undergo profound degeneration in monkeys chronically treated with low doses of MPTP. (2) In the caudate (head and body) nucleus of parkinsonian monkeys, there is an increased density of ChIs. (3) Despite the robust loss of CM/Pf neurons, no significant change was found in the density of thalamostriatal (vGluT2-positive) terminals, and in the prevalence of vGluT2-positive terminals in contact with ChIs in parkinsonian monkeys. These findings provide new information about the state of thalamic innervation of the striatum in parkinsonian monkeys with CM/Pf degeneration, and bring up an additional level of intricacy to the consequences of thalamic pathology upon the functional microcircuitry of the thalamostriatal system in parkinsonism. Future studies are needed to assess the importance of CM/Pf neuronal loss, and its potential consequences on the neuroplastic changes induced in the synaptic organization of the thalamostriatal system, in the development of early cognitive impairments in PD.
Keywords: Non-human primates; Parafascicular; Parkinson’s disease; Striatum; Thalamostriatal; vGluT2.
Conflict of interest statement
Figures





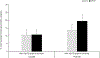
Similar articles
-
Neuronal loss in the caudal intralaminar thalamic nuclei in a primate model of Parkinson's disease.Brain Struct Funct. 2014 Jan;219(1):381-94. doi: 10.1007/s00429-013-0507-9. Epub 2013 Mar 19. Brain Struct Funct. 2014. PMID: 23508713 Free PMC article.
-
The thalamostriatal systems: anatomical and functional organization in normal and parkinsonian states.Brain Res Bull. 2009 Feb 16;78(2-3):60-8. doi: 10.1016/j.brainresbull.2008.08.015. Epub 2008 Sep 19. Brain Res Bull. 2009. PMID: 18805468 Free PMC article. Review.
-
Nigral and pallidal inputs to functionally segregated thalamostriatal neurons in the centromedian/parafascicular intralaminar nuclear complex in monkey.J Comp Neurol. 2002 Jun 3;447(3):286-99. doi: 10.1002/cne.10247. J Comp Neurol. 2002. PMID: 11984822
-
Effect of unilateral lesion of the nigrostriatal dopamine pathway on survival and neurochemistry of parafascicular nucleus neurons in the rat--evaluation of time-course and LGR8 expression.Brain Res. 2009 May 19;1271:83-94. doi: 10.1016/j.brainres.2009.03.026. Epub 2009 Mar 25. Brain Res. 2009. PMID: 19328193
-
Roles of centromedian parafascicular nuclei of thalamus and cholinergic interneurons in the dorsal striatum in associative learning of environmental events.J Neural Transm (Vienna). 2018 Mar;125(3):501-513. doi: 10.1007/s00702-017-1713-z. Epub 2017 Mar 21. J Neural Transm (Vienna). 2018. PMID: 28324169 Free PMC article. Review.
Cited by
-
Synaptic determinants of cholinergic interneurons hyperactivity during parkinsonism.Front Synaptic Neurosci. 2022 Sep 6;14:945816. doi: 10.3389/fnsyn.2022.945816. eCollection 2022. Front Synaptic Neurosci. 2022. PMID: 36147730 Free PMC article.
-
Motor cortical neuronal hyperexcitability associated with α-synuclein aggregation.NPJ Parkinsons Dis. 2025 Jan 15;11(1):18. doi: 10.1038/s41531-024-00867-z. NPJ Parkinsons Dis. 2025. PMID: 39809792 Free PMC article.
-
Movement-related activity in the internal globus pallidus of the parkinsonian macaque.J Neurophysiol. 2025 Aug 1;134(2):741-765. doi: 10.1152/jn.00611.2024. Epub 2025 Jul 22. J Neurophysiol. 2025. PMID: 40695735 Free PMC article.
-
Motor Cortical Neuronal Hyperexcitability Associated with α-Synuclein Aggregation.bioRxiv [Preprint]. 2024 Aug 14:2024.07.24.604995. doi: 10.1101/2024.07.24.604995. bioRxiv. 2024. Update in: NPJ Parkinsons Dis. 2025 Jan 15;11(1):18. doi: 10.1038/s41531-024-00867-z. PMID: 39091827 Free PMC article. Updated. Preprint.
-
Movement-related activity in the internal globus pallidus of the parkinsonian macaque.bioRxiv [Preprint]. 2025 May 30:2024.08.29.610310. doi: 10.1101/2024.08.29.610310. bioRxiv. 2025. Update in: J Neurophysiol. 2025 Aug 1;134(2):741-765. doi: 10.1152/jn.00611.2024. PMID: 39257740 Free PMC article. Updated. Preprint.
References
-
- Akins PT, Surmeier DJ, Kitai ST (1990) Muscarinic modulation of a transient K+ conductance in rat neostriatal neurons. Nature 344:240–242. - PubMed
-
- Altar CA, Heikkila RE, Manzino L, Marien MR (1986) 1-Methyl-4-phenylpyridine (MPP+): regional dopamine neuron uptake, toxicity, and novel rotational behavior following dopamine receptor proliferation. Eur J Pharmacol 131:199–209. - PubMed
-
- Aosaki T, Miura M, Suzuki T, Nishimura K, Masuda M (2010) Acetylcholine-dopamine balance hypothesis in the striatum: an update. Geriatr Gerontol Int 10 Suppl 1:S148–157. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

