Longitudinal analysis of quality of life following treatment with Asunercept plus reirradiation versus reirradiation in progressive glioblastoma patients
- PMID: 31679112
- PMCID: PMC6881251
- DOI: 10.1007/s11060-019-03320-x
Longitudinal analysis of quality of life following treatment with Asunercept plus reirradiation versus reirradiation in progressive glioblastoma patients
Abstract
Purpose: Glioblastoma is an aggressive malignant cancer of the central nervous system, with disease progression associated with deterioration of neurocognitive function and quality of life (QoL). As such, maintenance of QoL is an important treatment goal. This analysis presents time to deterioration (TtD) of QoL in patients with recurrent glioblastoma receiving Asunercept plus reirradiation (rRT) or rRT alone.
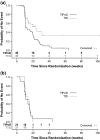
Methods: Data from patients with a baseline and ≥ 1 post-baseline QoL assessment were included in this analysis. TtD was defined as the time from randomisation to the first deterioration in the EORTC QLQ-C15, PAL EORTC QLQ-BN20 and Medical Research Council (MRC)-Neurological status. Deterioration was defined as a decrease of ≥ 10 points from baseline in the QLQ-C15 PAL overall QoL and functioning scales, an increase of ≥ 10 points from baseline in the QLQ-C15 PAL fatigue scale and the QLQ-BN20 total sum of score, and a rating of "Worse" in the MRC-Neurological status. Patients without a deterioration were censored at the last QoL assessment. Kaplan-Meier estimates were used to describe TtD and treatment groups (Asunercept + rRT or rRT alone) were compared using the log-rank test.
Results: Treatment with Asunercept + rRT was associated with significant improvement of TtD compared with rRT alone for QLQ-CL15 PAL overall QoL and physical functioning, and MRC Neurological Status (p ≤ 0.05). In the Asunercept + rRT group, QoL was maintained beyond progresison of disease (PoD).
Conclusion: Treatment with Asunercept plus rRT significantly prolongs TtD and maintains QoL versus rRT alone in recurrent glioblastoma patients.
Keywords: Asunercept; Quality of life; Recurrent glioblastoma; Time to deterioration.
Conflict of interest statement
Wolfgang Wick reports receiving a commercial research grant from Boehringer Ingelheim and Roche; speaker’s bureau honoraria from Prime Oncology; and is a consultant/advisory board member for Eli Lilly and Co. and Roche. Andriy Krendyukov and Thomas Höger are employees of Apogenix. No potential conflicts of interest were disclosed by Klaus Junge.
Figures

References
-
- Rock K, McArdle O, Forde P, et al. A clinical review of treatment outcomes in glioblastoma multiforme: the validation in a non-trial population of the results of a randomised phase III clinical trial: has a more radical approach improved survival? Br J Radiol. 2012;85:e729–e733. doi: 10.1259/bjr/83796755. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

