Development and First Use of the Patient's Qualitative Assessment of Treatment (PQAT) Questionnaire in Type 2 Diabetes Mellitus to Explore Individualised Benefit-Harm of Drugs Received During Clinical Studies
- PMID: 31679129
- PMCID: PMC7007419
- DOI: 10.1007/s40264-019-00877-4
Development and First Use of the Patient's Qualitative Assessment of Treatment (PQAT) Questionnaire in Type 2 Diabetes Mellitus to Explore Individualised Benefit-Harm of Drugs Received During Clinical Studies
Abstract
Introduction: Individualised benefit-harm assessments can help identify patient-perceived benefits and harms of a treatment, and associated trade-offs that may influence patients' willingness to use a treatment. This research presents the first use of a patient-reported outcome measure designed to assess patient-perceived benefits and disadvantages of drugs received during clinical studies.
Methods: The Patient's Qualitative Assessment of Treatment (PQAT) was developed in English and cognitively tested with US (n = 4) and Canadian (n = 3) patients with type 1 and type 2 diabetes mellitus (T2DM). The revised version of the PQAT comprises three qualitative open-ended questions focused on the benefits and disadvantages of treatment and reasons why patients would choose to continue/discontinue treatment. A final quantitative question asks patients to evaluate the balance between benefits and disadvantages using a 7-point scale. The revised version of the questionnaire was administered as an exploratory endpoint in a phase II clinical trial for a new injectable treatment for T2DM. Qualitative data were analysed using thematic analysis, and relationships between qualitative and quantitative data were identified.
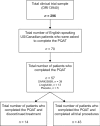
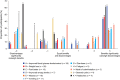
Results: Patient-reported benefits of treatment administered during the clinical trial included clinical markers of efficacy and subjective markers. Disadvantages reported by patients were mainly related to drug adverse effects or to the mode of administration. Of the 57 patients completing the PQAT, 70.2% reported being willing to continue treatment, with 59.6% reporting that the benefits outweighed the disadvantages. The reported benefits of feeling better and improved energy levels were more likely to be associated with a more positive ratio (70% and 71.4%, respectively), while the disadvantages of fatigue, headaches, and stomach pain were associated with a negative ratio and patients not being willing to continue the treatment.
Conclusions: The PQAT is a unique patient-reported outcome tool designed to aid understanding patients' real experience of benefits and disadvantages of a treatment. It combines the richness of qualitative data with quantitative data-information valuable for various stakeholders to make well-informed treatment decisions.
Trial registration: ClinicalTrials.gov identifier: NCT02973321.
Conflict of interest statement
Adam Gater, Amy Findley, and Kate Burrows are employed by Adelphi Values, which has received funding from Sanofi to analyse data reported in this paper. Aude Roborel de Climens, My-Liên Nguyên-Pascal, and Catherine Brun-Strang are paid employees of Sanofi. Aude Roborel de Climens and My-Liên Nguyên-Pascal are stockholders of Sanofi. Matthew Reaney was a paid employee and stockholder of Sanofi at the time of this study.
Figures



References
-
- Food and Drug Administration. The voice of the patient: a series of reports from FDA’s patient-focused drug development initiative. 2017. https://www.fda.gov/ForIndustry/UserFees/PrescriptionDrugUserFee/ucm3683.... Accessed 2 Apr 2019.
-
- Food and Drug Administration. Developing and submitting proposed draft guidance relating to patient experience data. 2019. https://www.fda.gov/drugs/development-approval-process-drugs/developing-.... Accessed 30 Apr 2019.
-
- Food and Drug Administration. Public workshop on patient-focused drug development: developing and submitting proposed draft guidance relating to patient experience data, 2018. https://www.fda.gov/drugs/news-events-human-drugs/public-workshop-patien.... Accessed 30 Apr 2019.
-
- Food and Drug Administration. CDER patient-focused drug development. 2018. https://www.fda.gov/drugs/development-approval-process-drugs/cder-patien.... Accessed 30 Apr 2019.
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical

