Bicuspid valve aortopathy is associated with distinct patterns of matrix degradation
- PMID: 31679706
- PMCID: PMC7674632
- DOI: 10.1016/j.jtcvs.2019.08.094
Bicuspid valve aortopathy is associated with distinct patterns of matrix degradation
Abstract
Objective: To explore the micromechanical, biochemical, and microstructural differences between bicuspid aortic valve aneurysm (BAV-A) and tricuspid aortic valve idiopathic degenerative aneurysm (DA), compared with normal aorta.
Methods: Aortic tissue was obtained from patients undergoing aneurysmal repair surgery (BAV-A; n = 15 and DA; n = 15). Control tissue was obtained from aortic punch biopsies during coronary artery bypass graft surgery (n = 9). Nanoindentation was used to determine the elastic modulus on the medial layer. Glycosaminoglycan, collagen, and elastin levels were measured using biochemical assays. Verhoeff Van Gieson-stained cross-sections were imaged for elastin microstructural quantification.
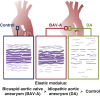
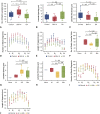
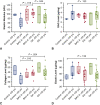
Results: The elastic modulus was more than 20% greater for BAV-A relative to control and DA (signifying a loss of compliance). No significance difference between control and DA were observed. Collagen levels for BAV-A (36.9 ± 7.4 μg/mg) and DA (49.9 ± 10.9 μg/mg) were greater compared with the control (30.2 ± 13.1 μg/mg). Glycosaminoglycan and elastin levels were not significant between the groups. Elastin segments were uniform throughout the control. Aneurysmal tissues had less elastin segments close to the intima and adventitia layers. Both BAV-A and DA had elastin segments compacted in the media; however, elastin segments were highly fragmented in DA.
Conclusions: BAV-A has a greater loss of aortic wall compliance relative to DA and the control. Although elastin levels were equal for all groups, spatial distribution of elastin provided a unique profile of matrix degradation for BAV-A. Elastin compaction within the media of BAV-A may have resulted from the altered hemodynamic pressure against the wall, which could explain for the stiffness of the tissue.
Keywords: bicuspid aortic valve aortopathy; biochemistry; elastin; idiopathic degenerative aneurysms; micromechanics; microstructure.
Copyright © 2019 The American Association for Thoracic Surgery. Published by Elsevier Inc. All rights reserved.
Figures








Comment in
-
Commentary: Can we move beyond aortic size, using real-time analysis of aortic tissue, to more precisely guide therapy for patients with bicuspid aortic valves?J Thorac Cardiovasc Surg. 2020 Dec;160(6):e259. doi: 10.1016/j.jtcvs.2019.09.014. Epub 2019 Sep 20. J Thorac Cardiovasc Surg. 2020. PMID: 31607498 No abstract available.
-
Commentary: Aortic aneurysms are not created equal.J Thorac Cardiovasc Surg. 2020 Dec;160(6):e261-e262. doi: 10.1016/j.jtcvs.2019.09.013. Epub 2019 Sep 20. J Thorac Cardiovasc Surg. 2020. PMID: 31619330 No abstract available.
References
-
- Hope M.D., Hope T.A., Meadows A.K., Ordovas K.G., Urbania T.H., Alley M.T. Bicuspid aortic valve: four-dimensional MR evaluation of ascending aortic systolic flow patterns. Radiology. 2010;255:53–61. - PubMed
-
- Shahmansouri N., Alreshidan M., Emmott A., Lachapelle K., Cartier R., Leask R.L. Evaluating ascending aortic aneurysm tissue toughness: dependence on collagen and elastin contents. J Mech Behav Biomed Mater. 2016;64:262–271. - PubMed
E-References
-
- Chow M.J., Zhang Y. Changes in the mechanical and biochemical properties of aortic tissue due to cold storage. J Surg Res. 2011;171:434–442. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

