Meiotic Kinetochores Fragment into Multiple Lobes upon Cohesin Loss in Aging Eggs
- PMID: 31679939
- PMCID: PMC6868511
- DOI: 10.1016/j.cub.2019.09.006
Meiotic Kinetochores Fragment into Multiple Lobes upon Cohesin Loss in Aging Eggs
Abstract
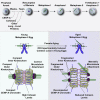
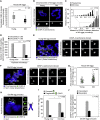
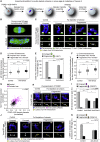
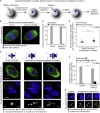
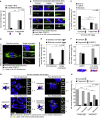
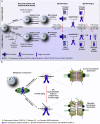
Chromosome segregation errors during female meiosis are a leading cause of pregnancy loss and human infertility. The segregation of chromosomes is driven by interactions between spindle microtubules and kinetochores. Kinetochores in mammalian oocytes are subjected to special challenges: they need to withstand microtubule pulling forces over multiple hours and are built on centromeric chromatin that in humans is decades old. In meiosis I, sister kinetochores are paired and oriented toward the same spindle pole. It is well established that they progressively separate from each other with advancing female age. However, whether aging also affects the internal architecture of centromeres and kinetochores is currently unclear. Here, we used super-resolution microscopy to study meiotic centromere and kinetochore organization in metaphase-II-arrested eggs from three mammalian species, including humans. We found that centromeric chromatin decompacts with advancing maternal age. Kinetochores built on decompacted centromeres frequently lost their integrity and fragmented into multiple lobes. Fragmentation extended across inner and outer kinetochore regions and affected over 30% of metaphase-II-arrested (MII) kinetochores in aged women and mice, making the lobular architecture a prominent feature of the female meiotic kinetochore. We demonstrate that a partial cohesin loss, as is known to occur in oocytes with advancing maternal age, is sufficient to trigger centromere decompaction and kinetochore fragmentation. Microtubule pulling forces further enhanced the fragmentation and shaped the arrangement of kinetochore lobes. Fragmented kinetochores were frequently abnormally attached to spindle microtubules, suggesting that kinetochore fragmentation could contribute to the maternal age effect in mammalian eggs.
Keywords: Trim-Away; aging; aneuploidy; centromere; cohesion; human; kinetochore; maternal age effect; meiosis; oocyte.
Copyright © 2019 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Black B.E., Bassett E.A. The histone variant CENP-A and centromere specification. Curr. Opin. Cell Biol. 2008;20:91–100. - PubMed
-
- Pesenti M.E., Weir J.R., Musacchio A. Progress in the structural and functional characterization of kinetochores. Curr. Opin. Struct. Biol. 2016;37:152–163. - PubMed
-
- Pereira A.J., Maiato H. Maturation of the kinetochore-microtubule interface and the meaning of metaphase. Chromosome Res. 2012;20:563–577. - PubMed
-
- Auckland P., McAinsh A.D. Building an integrated model of chromosome congression. J. Cell Sci. 2015;128:3363–3374. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

