6-Substituted amiloride derivatives as inhibitors of the urokinase-type plasminogen activator for use in metastatic disease
- PMID: 31679971
- PMCID: PMC7362394
- DOI: 10.1016/j.bmcl.2019.126753
6-Substituted amiloride derivatives as inhibitors of the urokinase-type plasminogen activator for use in metastatic disease
Abstract
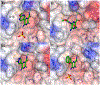
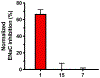
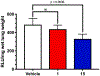
The oral K+-sparing diuretic amiloride shows anti-cancer side-activities in multiple rodent models. These effects appear to arise, at least in part, through moderate inhibition of the urokinase-type plasminogen activator (uPA, Ki = 2.4 µM), a pro-metastatic trypsin-like serine protease that is upregulated in many aggressive solid malignancies. In applying the selective optimization of side-activity (SOSA) approach, a focused library of twenty two 6-substituted amiloride derivatives were prepared, with multiple examples displaying uPA inhibitory potencies in the nM range. X-ray co-crystal structures revealed that the potency increases relative to amiloride arise from increased occupancy of uPA's S1β subsite by the appended 6-substituents. Leading compounds were shown to have high selectivity over related trypsin-like serine proteases and no diuretic or anti-kaliuretic effects in rats. Compound 15 showed anti-metastatic effects in a xenografted mouse model of late-stage lung metastasis.
Keywords: Amiloride; Anti-metastatic; Cancer; Metastasis; Selective optimisation of side-activity; Trypsin-like serine protease; Urokinase plasminogen activator; uPA.
Copyright © 2019 Elsevier Ltd. All rights reserved.
Figures




References
-
- Lant AF, Smith AJ, Wilson GM. Clinical evaluation of amiloride, a potassium-sparing diuretic. Clin Pharmacol Ther. 1969;10(1):50–63. - PubMed
-
- Cragoe EJJ, Woltersdorf OWJ, Bicking JB, Kwong SF, Jones JH. Pyrazine diuretics. II. N-amidino-3-amino-5-substituted 6-halopyrazinecarboxamides. J Med Chem. 1967;10(1):66–75. - PubMed
-
- Kleyman TR, Cragoe EJJ. The mechanism of action of amiloride. Sem Nephrol. 1988;8(3):242–248. - PubMed
-
- Matthews H, Ranson M, Kelso MJ. Anti-tumour/metastasis effects of the potassium-sparing diuretic amiloride: an orally active anti-cancer drug waiting for its call-of-duty? Int J Cancer. 2011;129(9):2051–2061. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Chemical Information
Miscellaneous

