ELM-the eukaryotic linear motif resource in 2020
- PMID: 31680160
- PMCID: PMC7145657
- DOI: 10.1093/nar/gkz1030
ELM-the eukaryotic linear motif resource in 2020
Abstract
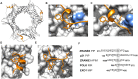
The eukaryotic linear motif (ELM) resource is a repository of manually curated experimentally validated short linear motifs (SLiMs). Since the initial release almost 20 years ago, ELM has become an indispensable resource for the molecular biology community for investigating functional regions in many proteins. In this update, we have added 21 novel motif classes, made major revisions to 12 motif classes and added >400 new instances mostly focused on DNA damage, the cytoskeleton, SH2-binding phosphotyrosine motifs and motif mimicry by pathogenic bacterial effector proteins. The current release of the ELM database contains 289 motif classes and 3523 individual protein motif instances manually curated from 3467 scientific publications. ELM is available at: http://elm.eu.org.
© The Author(s) 2019. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures


References
-
- Davey N.E., Van Roey K., Weatheritt R.J., Toedt G., Uyar B., Altenberg B., Budd A., Diella F., Dinkel H., Gibson T.J.. Attributes of short linear motifs. Mol. Biosyst. 2012; 8:268–281. - PubMed
-
- Van Roey K., Uyar B., Weatheritt R.J., Dinkel H., Seiler M., Budd A., Gibson T.J., Davey N.E.. Short linear motifs: ubiquitous and functionally diverse protein interaction modules directing cell regulation. Chem. Rev. 2014; 114:6733–6778. - PubMed
-
- Hunt T. Protein sequence motifs involved in recognition and targeting: a new series. Trends Biochem. Sci. 1990; 15:305. - PubMed
-
- Tompa P., Davey N.E., Gibson T.J., Babu M.M.. A million peptide motifs for the molecular biologist. Mol. Cell. 2014; 55:161–169. - PubMed
-
- Van Roey K., Gibson T.J., Davey N.E.. Motif switches: decision-making in cell regulation. Curr. Opin. Struct. Biol. 2012; 22:378–385. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

