Zearalenone and ß-Zearalenol But Not Their Glucosides Inhibit Heat Shock Protein 90 ATPase Activity
- PMID: 31680951
- PMCID: PMC6813925
- DOI: 10.3389/fphar.2019.01160
Zearalenone and ß-Zearalenol But Not Their Glucosides Inhibit Heat Shock Protein 90 ATPase Activity
Abstract
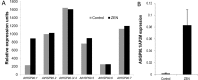
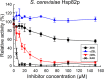
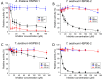
The mycotoxin zearalenone (ZEN) is produced by many plant pathogenic Fusarium species. It is well known for its estrogenic activity in humans and animals, but whether ZEN has a role in plant-pathogen interaction and which process it is targeting in planta was so far unclear. We found that treatment of Arabidopsis thaliana seedlings with ZEN induced transcription of the AtHSP90.1 gene. This heat shock protein (HSP) plays an important role in plant-pathogen interaction, assisting in stability and functionality of various disease resistance gene products. Inhibition of HSP90 ATPase activity impairs functionality. Because HSP90 inhibitors are known to induce HSP90 gene expression and due to the structural similarity with the known HSP90 inhibitor radicicol (RAD), we tested whether ZEN and its phase I metabolites α- and ß-zearalenol are also HSP90 ATPase inhibitors. Indeed, AtHSP90.1 and wheat TaHSP90-2 were inhibited by ZEN and ß-zearalenol, while α-zearalenol was almost inactive. Plants can efficiently glycosylate ZEN and α/ß-zearalenol. We therefore tested whether glucosylation has an effect on the inhibitory activity of these metabolites. Expression of the A. thaliana glucosyltransferase UGT73C6 conferred RAD resistance to a sensitive yeast strain. Glucosylation of RAD, ZEN, and α/ß-zearalenol abolished the in vitro inhibitory activity with recombinant HSP90 purified from Escherichia coli. In conclusion, the mycotoxin ZEN has a very prominent target in plants, HSP90, but it can be inactivated by glycosylation. This may explain why there is little evidence for a virulence function of ZEN in host plants.
Keywords: Arabidopsis; Fusarium; HSP90; glycosylation; radicicol; wheat.
Copyright © 2019 Torres Acosta, Michlmayr, Shams, Schweiger, Wiesenberger, Mitterbauer, Werner, Merz, Hauser, Hametner, Varga, Krska, Berthiller and Adam.
Figures



References
-
- Aoki T., Vaughan M. M., McCormick S. P., Busman M., Ward T. J., Kelly A., et al. (2015). Fusarium dactylidis sp. nov., a novel nivalenol toxin–producing species sister to F. pseudograminearum isolated from orchard grass (Dactylis glomerata) in Oregon and New Zealand. Mycologia 107, 409–418. 10.3852/14-213 - DOI - PubMed
-
- Berthiller F., Hametner C., Krenn P., Schweiger W., Ludwig R., Adam G., et al. (2009. a). Preparation and characterization of the conjugated Fusarium mycotoxins zearalenone-4 O-β-D-glucopyranoside, α-zearalenol-4 O-β-D-glucopyranoside and β-zearalenol-4 O-β-D-glucopyranoside by MS/MS and two-dimensional NMR. Food Addit. Contam. 26, 207–213. 10.1080/02652030802399034 - DOI - PubMed
-
- Berthiller F., Werner U., Sulyok M., Krska R., Hauser M. T., Schuhmacher R. (2006). Liquid chromatography coupled to tandem mass spectrometry (LC–MS/MS) determination of phase II metabolites of the mycotoxin zearalenone in the model plant Arabidopsis thaliana . Food Addit. Contam. 23, 1194–1200. 10.1080/02652030600778728 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

