Acute MR-Guided High-Intensity Focused Ultrasound Lesion Assessment Using Diffusion-Weighted Imaging and Histological Analysis
- PMID: 31681145
- PMCID: PMC6803785
- DOI: 10.3389/fneur.2019.01069
Acute MR-Guided High-Intensity Focused Ultrasound Lesion Assessment Using Diffusion-Weighted Imaging and Histological Analysis
Abstract
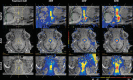
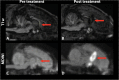
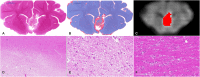
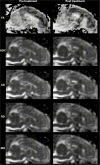
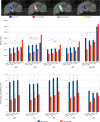
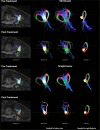
Objectives: The application of magnetic resonance-guided focused ultrasound (MRgFUS) for the treatment of neurological conditions has been of increasing interest. Conventional MR imaging can provide structural information about the effect of MRgFUS, where differences in ablated tissue can be seen, but it lacks information about the status of the cellular environment or neural microstructure. We investigate in vivo acute changes in water diffusion and white matter tracts in the brain of a piglet model after MRgFUS treatment using diffusion-weighted imaging (DWI) with histological verification of treatment-related changes. Methods: MRgFUS was used to treat the anterior body of the fornix in four piglets. T1 and diffusion-weighted images were collected before and after treatment. Mean diffusion-weighted imaging (MDWI) images were generated to measure lesion volumes via signal intensity thresholds. Histological data were collected for volume comparison and assessment of treatment effect. DWI metric maps of fractional anisotropy (FA), apparent diffusion coefficient (ADC), axial diffusivity (AD), radial diffusivity (RD), and mean diffusivity (MD) were generated for quantitative assessment. Fornix-related fiber tracts were generated before and after treatment for qualitative assessment. Results: The volume of treated tissue measured via MDWI did not differ significantly from histological measurements, and both were significantly larger than the treatment cell volume. Diffusion metrics in the treatment region were significantly decreased following MRgFUS treatment, with the peak change seen at the lesion core and decreasing radially. Histological analysis confirmed an area of coagulative necrosis in the targeted region with sharp demarcation zone with surrounding brain. Tractography from the lesion core and the fornix revealed fiber disruptions following treatment. Conclusions: Diffusion maps and fiber tractography are an effective method for assessing lesion volumes and microstructural changes in vivo following MRgFUS treatment. This study demonstrates that DWI has the potential to advance MRgFUS by providing convenient in vivo microstructural lesion and fiber tractography assessment after treatment.
Keywords: diffusion tensor imaging (DTI); diffusion weighted imaging (DWI); focused ultrasound; magnetic resonance-guided focused ultrasound (MRgFUS); tractography.
Copyright © 2019 Walker, Zhong, Waspe, Looi, Piorkowska, Hawkins, Drake and Hodaie.
Figures
References
LinkOut - more resources
Full Text Sources
Miscellaneous

