Successful Host Adaptation of IncK2 Plasmids
- PMID: 31681238
- PMCID: PMC6803427
- DOI: 10.3389/fmicb.2019.02384
Successful Host Adaptation of IncK2 Plasmids
Abstract
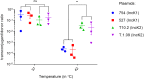
The IncK plasmid group can be divided into two separate lineages named IncK1 and IncK2. IncK2 is found predominantly in poultry while IncK1 was reported in various mammals, including animals and humans. The physiological basis of this distinction is not known. In this manuscript we examined fitness cost of IncK1 and IncK2 plasmids at 37 and 42°C, which resembles mammalian and chicken body temperatures, respectively. We analyzed conjugation frequency, plasmid copy number and plasmid fitness cost in direct competition. Additionally, we measured levels of σ-32 in Escherichia coli carrying either wild type or conjugation-deficient IncK plasmids. The results show that IncK2 plasmids have a higher conjugation frequency and lower copy number at 42°C compared to IncK1. While the overall fitness cost to the host bacterium of IncK2 plasmids was higher than that of IncK1, it was not affected by the temperature while the fitness cost of IncK1 was shown to increase at 42°C compared to 37°C. These differences correlate with an increased expression of σ-32, a regulator of heat-shock protein expression, in E. coli with IncK2 compared to cells containing IncK1. This effect was not seen in cells containing conjugation deficient plasmids. Therefore, it is hypothesized that the assembly of the functional T4S may lead to these increased levels of σ-32. Increased activation of CpxR at 42°C may explain why IncK2 plasmids, and not IncK1, are predominantly found in chicken isolates.
Keywords: IncK2; chicken; conjugation; plasmid; sigma-32.
Copyright © 2019 Rozwandowicz, Brouwer, Mughini-Gras, Wagenaar, Gonzalez-Zorn, Mevius and Hordijk.
Figures




References
LinkOut - more resources
Full Text Sources

