Engineered T Cell Therapy for Cancer in the Clinic
- PMID: 31681259
- PMCID: PMC6798078
- DOI: 10.3389/fimmu.2019.02250
Engineered T Cell Therapy for Cancer in the Clinic
Abstract
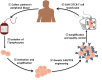
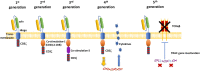
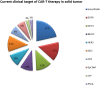
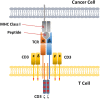
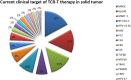
T cells play a key role in cell-mediated immunity, and strategies to genetically modify T cells, including chimeric antigen receptor (CAR) T cell therapy and T cell receptor (TCR) T cell therapy, have achieved substantial advances in the treatment of malignant tumors. In clinical trials, CAR-T cell and TCR-T cell therapies have produced encouraging clinical outcomes, thereby demonstrating their therapeutic potential in mitigating tumor development. This article summarizes the current applications of CAR-T cell and TCR-T cell therapies in clinical trials worldwide. It is predicted that genetically engineered T cell immunotherapies will become safe, well-tolerated, and effective therapeutics and bring hope to cancer patients.
Keywords: CAR-T cell; TCR-T cell; clinic; engineered T cells; immunotherapy.
Copyright © 2019 Zhao and Cao.
Figures

References
-
- Hendifar AE, Hingorani SR, Harris WP, Seery TE, Sigal D, Wu W, et al. LBA-02 high response rate and PFS with PEGPH20 added to Nab-Paclitaxel/Gemcitabine in stage IV previously untreated pancreatic cancer patients with high-HA tumors: interim results of a randomized phase 2 study. Ann Oncol. (2015) 26(Suppl. 4):iv117 10.1093/annonc/mdv262.02 - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

