Engineering the Yeast Saccharomyces cerevisiae for the Production of L-(+)-Ergothioneine
- PMID: 31681742
- PMCID: PMC6797849
- DOI: 10.3389/fbioe.2019.00262
Engineering the Yeast Saccharomyces cerevisiae for the Production of L-(+)-Ergothioneine
Abstract
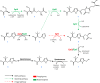
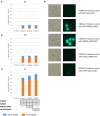
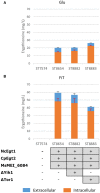
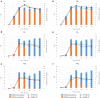
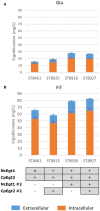
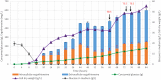
L-(+)-Ergothioneine (ERG) is an unusual, naturally occurring antioxidant nutraceutical that has been shown to help reduce cellular oxidative damage. Humans do not biosynthesise ERG, but acquire it from their diet; it exploits a specific transporter (SLC22A4) for its uptake. ERG is considered to be a nutraceutical and possible vitamin that is involved in the maintenance of health, and seems to be at too low a concentration in several diseases in vivo. Ergothioneine is thus a potentially useful dietary supplement. Present methods of commercial production rely on extraction from natural sources or on chemical synthesis. Here we describe the engineering of the baker's yeast Saccharomyces cerevisiae to produce ergothioneine by fermentation in defined media. After integrating combinations of ERG biosynthetic pathways from different organisms, we screened yeast strains for their production of ERG. The highest-producing strain was also engineered with known ergothioneine transporters. The effect of amino acid supplementation of the medium was investigated and the nitrogen metabolism of S. cerevisiae was altered by knock-out of TOR1 or YIH1. We also optimized the media composition using fractional factorial methods. Our optimal strategy led to a titer of 598 ± 18 mg/L ergothioneine in fed-batch culture in 1 L bioreactors. Because S. cerevisiae is a GRAS ("generally recognized as safe") organism that is widely used for nutraceutical production, this work provides a promising process for the biosynthetic production of ERG.
Keywords: Saccharomyces cerevisiae; ergothioneine; medium optimization; metabolic engineering; nutraceutical; yeast.
Copyright © 2019 van der Hoek, Darbani, Zugaj, Prabhala, Biron, Randelovic, Medina, Kell and Borodina.
Figures

References
-
- Audley B. G., Tan C. H. (1968). The uptake of ergothioneine from the soil into the latex of Hevea brasiliensis. Phytochemistry 7, 1999–2000. 10.1016/S0031-9422(00)90759-3 - DOI
-
- Barger G., Ewins A. J. (1911). CCLVII.—the constitution of ergothioneine: a betaine related to histidine. J. Chem. Soc. Trans. 99, 2336–2341. 10.1039/CT9119902336 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

