Electroactive Smart Materials: Novel Tools for Tailoring Bacteria Behavior and Fight Antimicrobial Resistance
- PMID: 31681752
- PMCID: PMC6813912
- DOI: 10.3389/fbioe.2019.00277
Electroactive Smart Materials: Novel Tools for Tailoring Bacteria Behavior and Fight Antimicrobial Resistance
Abstract
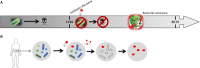
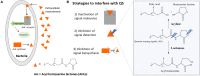
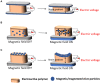
Despite being very simple organisms, bacteria possess an outstanding ability to adapt to different environments. Their long evolutionary history, being exposed to vastly different physicochemical surroundings, allowed them to detect and respond to a wide range of signals including biochemical, mechanical, electrical, and magnetic ones. Taking into consideration their adapting mechanisms, it is expected that novel materials able to provide bacteria with specific stimuli in a biomimetic context may tailor their behavior and make them suitable for specific applications in terms of anti-microbial and pro-microbial approaches. This review maintains that electroactive smart materials will be a future approach to be explored in microbiology to obtain novel strategies for fighting the emergence of live threatening antibiotic resistance.
Keywords: antimicrobial resistance; bacteria; biomimetics; electroactive materials; physical stimuli.
Copyright © 2019 Fernandes, Carvalho and Lanceros-Mendez.
Figures



References
-
- Amaro L., Correia D. M., Marques-Almeida T., Martins P. M., Pérez L., Vilas J. L., et al. . (2018). Tailored biodegradable and electroactive poly(Hydroxybutyrate-co-hydroxyvalerate) based morphologies for tissue engineering applications. Int. J. Mol. Sci. 19:2149. 10.3390/ijms19082149 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

