Safety, Tolerability, and Pharmacokinetics of the Broadly Neutralizing Human Immunodeficiency Virus (HIV)-1 Monoclonal Antibody VRC01 in HIV-Exposed Newborn Infants
- PMID: 31681963
- PMCID: PMC7377284
- DOI: 10.1093/infdis/jiz532
Safety, Tolerability, and Pharmacokinetics of the Broadly Neutralizing Human Immunodeficiency Virus (HIV)-1 Monoclonal Antibody VRC01 in HIV-Exposed Newborn Infants
Abstract
Background: Although mother-to-child human immunodeficiency virus (HIV) transmission has dramatically decreased with maternal antiretroviral therapy, breast milk transmission accounts for most of the 180 000 new infant HIV infections annually. Broadly neutralizing antibodies (bNAb) may further reduce transmission.
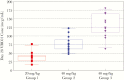
Methods: A Phase 1 safety and pharmacokinetic study was conducted: a single subcutaneous (SC) dose of 20 or 40 mg/kg (Dose Groups 1 and 2, respectively) of the bNAb VRC01 was administered to HIV-exposed infants soon after birth. Breastfeeding infants (Dose Group 3) received 40 mg/kg SC VRC01 after birth and then 20 mg/kg/dose SC monthly. All infants received appropriate antiretroviral prophylaxis.
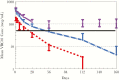
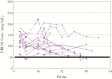
Results: Forty infants were enrolled (21 in the United States, 19 in Africa). Subcutaneous VRC01 was safe and well tolerated with only mild-to-moderate local reactions, primarily erythema, which rapidly resolved. For multiple-dose infants, local reactions decreased with subsequent injections. VRC01 was rapidly absorbed after administration, with peak concentrations 1-6 days postdose. The 40 mg/kg dose resulted in 13 of 14 infants achieving the serum 50 micrograms (mcg)/mL target at day 28. Dose Group 3 infants maintained concentrations greater than 50 mcg/mL throughout breastfeeding.
Conclusions: Subcutaneous VRC01 as single or multiple doses is safe and well tolerated in very young infants and is suitable for further study to prevent HIV transmission in infants.
Keywords: HIV-1; VRC01; broadly neutralizing antibodies; mother-to-child transmission of HIV; neonates.
© The Author(s) 2019. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail: journals.permissions@oup.com.
Figures
Comment in
-
Broadly Neutralizing Antihuman Immunodeficiency Virus Antibodies in Infants: Promising New Tools for Prevention of Mother-to-Child Transmission?J Infect Dis. 2020 Jul 23;222(4):525-527. doi: 10.1093/infdis/jiz536. J Infect Dis. 2020. PMID: 31681956 No abstract available.

