Clinical utility of hereditary cancer panel testing: Impact of PALB2, ATM, CHEK2, NBN, BRIP1, RAD51C, and RAD51D results on patient management and adherence to provider recommendations
- PMID: 31682005
- PMCID: PMC7003834
- DOI: 10.1002/cncr.32572
Clinical utility of hereditary cancer panel testing: Impact of PALB2, ATM, CHEK2, NBN, BRIP1, RAD51C, and RAD51D results on patient management and adherence to provider recommendations
Abstract
Background: Although management guidelines exist for several genes associated with a 2-fold to 5-fold increase in the relative risk for certain cancers, the value of testing for them remains controversial.
Methods: De-identified personal and family history data for 654 individuals with pathogenic variants (PVs) in PALB2, ATM, CHEK2, NBN, BRIP1, RAD51C, and/or RAD51D were analyzed for pretest and post-test candidacy for guideline-recommended management of cancer risk. These individuals were invited to complete a survey about provider recommendations and their adherence.
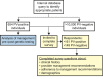
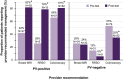
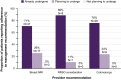
Results: Twenty-four percent of CHEK2, ATM, PALB2, or NBN PV carriers were appropriate for consideration of annual breast magnetic resonance imaging screening before genetic testing, with the remaining 76% appropriate only after testing. No BRIP1, RAD51C, or RAD51D PV carriers were appropriate for consideration of risk-reducing salpingo-oophorectomy before genetic testing; 100% were appropriate only after testing. Seventeen percent of CHEK2 PV carriers were appropriate for earlier and more frequent colonoscopy before genetic testing, with the remaining 83% appropriate only after testing. Provider recommendations for annual breast magnetic resonance imaging, consideration of risk-reducing salpingo-oophorectomy, and earlier and more frequent colonoscopy were reported by 42%, 26%, and 66% of breast, ovarian, and colorectal cancer risk PV carriers, respectively, before genetic testing, versus 82%, 79%, and 81%, respectively, after testing. Nearly all respondents had planned or undertaken provider-recommended management.
Conclusions: Testing for PALB2, ATM, CHEK2, NBN, BRIP1, RAD51C, and RAD51D changed management for those carrying PVs. Provider recommendations were aligned with guidelines, and patients adhered to recommendations, both of which are critical for reducing both long-term cancer morbidity and mortality.
Keywords: breast cancer; cancer risk; colorectal cancer; hereditary cancer; ovarian cancer.
© 2019 Myriad Genetics, Inc. Cancer published by Wiley Periodicals, Inc. on behalf of American Cancer Society.
Conflict of interest statement
All authors are current or former employees of Myriad Genetics, Inc, Counsyl, Inc, and/or Myriad Women's Health.
Figures
References
-
- National Comprehensive Cancer Network (NCCN) . NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Genetic/Familial High‐Risk Assessment: Breast and Ovarian. Version 2.2019. NCCN; 2019.
-
- National Comprehensive Cancer Network (NCCN) . NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Genetic/Familial High‐Risk Assessment: Colorectal. Version 1.2018. NCCN; 2018.
-
- Hall MJ, Forman AD, Pilarski R, Wiesner G, Giri VN. Gene panel testing for inherited cancer risk. J Natl Compr Canc Netw. 2014;12:1339‐1346. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

