Intranasal midazolam as first-line inhospital treatment for status epilepticus: a pharmaco-EEG cohort study
- PMID: 31682078
- PMCID: PMC6917318
- DOI: 10.1002/acn3.50932
Intranasal midazolam as first-line inhospital treatment for status epilepticus: a pharmaco-EEG cohort study
Abstract
Objective: We sought to evaluate the efficacy and tolerability of intranasal midazolam (in-MDZ) as first-line inhospital therapy in patients with status epilepticus (SE) during continuous EEG recording.
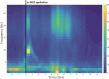
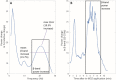
Methods: Data on medical history, etiology and semiology of SE, anticonvulsive medication usage, efficacy and safety of in-MDZ were retrospectively reviewed between 2015 and 2018. Time to end of SE regarding the administration of in-MDZ and ß-band effects were analyzed on EEG and with frequency analysis.
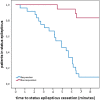
Results: In total, 42 patients (mean age: 52.7 ± 22.7 years; 23 females) were treated with a median dose of 5 mg of in-MDZ (range: 2.5-15 mg, mean: 6.4 mg, SD: 2.6) for SE. The majority of the patients suffered from nonconvulsive SE (n = 24; 55.8%). In total, 24 (57.1%) patients were responders, as SE stopped following the administration of in-MDZ without any other drugs being given. On average, SE ceased on EEG at 05:05 (minutes:seconds) after the application of in-MDZ (median: 04:56; range: 00:29-14:53; SD:03:13). Frequency analysis showed an increased ß-band on EEG after the application of in-MDZ at 04:07 on average (median: 03:50; range: 02:20-05:40; SD: 01:09). Adverse events were recorded in six patients (14.3%), with nasal irritations present in five (11.9%) and prolonged sedation occurring in one (2.6%) patient.
Conclusions: This pharmaco-EEG-based study showed that in-MDZ is effective and well-tolerated for the acute treatment of SE. EEG and clinical effects of in-MDZ administration occurred within 04:07 and 5:05 on average. Intranasal midazolam appears to be an easily applicable and rapidly effective alternative to buccal or intramuscular application as first-line treatment if an intravenous route is not available.
© 2019 The Authors. Annals of Clinical and Translational Neurology published by Wiley Periodicals, Inc on behalf of American Neurological Association.
Conflict of interest statement
Dr. Kay reports nonfinancial support from Eisai, nonfinancial support from UCB, outside the submitted work. Dr. Merkel has nothing to disclose. A. von Blomberg has nothing to disclose. Dr. Bauer has nothing to disclose. Dr. Willems has nothing to disclose. Dr. Reif received personal fees from Eisai, outside the submitted work. Dr. Schubert‐Bast reports personal fees from UCB Pharma, Eisai, Desitin Pharma, LivaNova, and Zogenix, outside the scope of the submitted work. Dr. Rosenow reports personal fees and nonfinancial support from UCB Pharma, personal fees from Shire, personal fees from EISAI, personal fees from Desitin Arzneimittel, personal fees from Bial, personal fees from cerbomed, nonfinancial support from Novartis Japan, personal fees from Bayer Vital, personal fees from Sandoz, personal fees from Verband der forschenden Arzneimittleindustrie, grants from European Union, FP7, grants from Hessisches Ministerium für Wissenschaft und Kunst (LOEWE‐Programme), personal fees from GW Pharma, grants from Detlev‐Wrobel Fonds for Epilepsy Research, outside the submitted work. Dr. Strzelczyk reports grants and personal fees from Desitin Arzneimittel, personal fees from Eisai, grants and personal fees from GW pharmaceuticals, grants and personal fees from LivaNova, personal fees from Marinus pharmaceuticals, personal fees from Medtronic, grants and personal fees from Sage therapeutics, grants and personal fees from UCB pharma, grants and personal fees from Zogenix, outside the submitted work.
Figures
Similar articles
-
Efficacy, Tolerability, and Safety of Concentrated Intranasal Midazolam Spray as Emergency Medication in Epilepsy Patients During Video-EEG Monitoring.CNS Drugs. 2020 May;34(5):545-553. doi: 10.1007/s40263-020-00720-w. CNS Drugs. 2020. PMID: 32219682 Free PMC article.
-
Continuous EEG monitoring and midazolam infusion for refractory nonconvulsive status epilepticus.Neurology. 2001 Sep 25;57(6):1036-42. doi: 10.1212/wnl.57.6.1036. Neurology. 2001. PMID: 11571331
-
Intranasal Midazolam versus Rectal Diazepam for the Management of Canine Status Epilepticus: A Multicenter Randomized Parallel-Group Clinical Trial.J Vet Intern Med. 2017 Jul;31(4):1149-1158. doi: 10.1111/jvim.14734. Epub 2017 May 24. J Vet Intern Med. 2017. PMID: 28543780 Free PMC article. Clinical Trial.
-
Intramuscular Midazolam for treatment of Status Epilepticus.Expert Opin Pharmacother. 2021 Jan;22(1):37-44. doi: 10.1080/14656566.2020.1810236. Epub 2020 Aug 25. Expert Opin Pharmacother. 2021. PMID: 32840150 Review.
-
A Common Reference-Based Indirect Comparison Meta-Analysis of Buccal versus Intranasal Midazolam for Early Status Epilepticus.CNS Drugs. 2015 Sep;29(9):741-57. doi: 10.1007/s40263-015-0271-x. CNS Drugs. 2015. PMID: 26293745 Review.
Cited by
-
The nose has it: Opportunities and challenges for intranasal drug administration for neurologic conditions including seizure clusters.Epilepsy Behav Rep. 2022 Dec 28;21:100581. doi: 10.1016/j.ebr.2022.100581. eCollection 2023. Epilepsy Behav Rep. 2022. PMID: 36636458 Free PMC article. Review.
-
Opportunities for and Challenges of Pulmonary Drug Delivery in the Management of Acute Exacerbations of CNS Disorders.CNS Drugs. 2025 Aug 7. doi: 10.1007/s40263-025-01213-4. Online ahead of print. CNS Drugs. 2025. PMID: 40775197 Review.
-
[S2k guidelines: status epilepticus in adulthood : Guidelines of the German Society for Neurology].Nervenarzt. 2021 Oct;92(10):1002-1030. doi: 10.1007/s00115-020-01036-2. Epub 2021 Mar 22. Nervenarzt. 2021. PMID: 33751150 Free PMC article. Review. German.
-
Efficacy, Tolerability, and Safety of Concentrated Intranasal Midazolam Spray as Emergency Medication in Epilepsy Patients During Video-EEG Monitoring.CNS Drugs. 2020 May;34(5):545-553. doi: 10.1007/s40263-020-00720-w. CNS Drugs. 2020. PMID: 32219682 Free PMC article.
-
Why won't it stop? The dynamics of benzodiazepine resistance in status epilepticus.Nat Rev Neurol. 2022 Jul;18(7):428-441. doi: 10.1038/s41582-022-00664-3. Epub 2022 May 10. Nat Rev Neurol. 2022. PMID: 35538233 Review.
References
-
- Strzelczyk A, Ansorge S, Hapfelmeier J, et al. Costs, length of stay, and mortality of super‐refractory status epilepticus: a population‐based study from Germany. Epilepsia 2017;58:1533–1541. - PubMed
-
- Knake S, Rosenow F, Vescovi M, et al. Incidence of status epilepticus in adults in Germany: a prospective, population‐based study. Epilepsia 2001;42:714–718. - PubMed
-
- Schubert‐Bast S, Zöllner JP, Ansorge S, et al. Burden and epidemiology of status epilepticus in infants, children, and adolescents: a population‐based study on German health insurance data. Epilepsia 2019;60:911–920. - PubMed
-
- Neligan A, Shorvon SD. Frequency and prognosis of convulsive status epilepticus of different causes: a systematic review. Arch Neurol 2010;67:931–940. - PubMed
-
- Shorvon S. Super‐refractory status epilepticus: an approach to therapy in this difficult clinical situation. Epilepsia 2011;52(Suppl 8):53–56. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
