Molecular and cellular physiology of organic cation transporter 2
- PMID: 31682169
- PMCID: PMC6962513
- DOI: 10.1152/ajprenal.00422.2019
Molecular and cellular physiology of organic cation transporter 2
Abstract
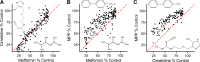
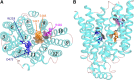
Organic cation transporters play a critical role in mediating the distribution of cationic pharmaceuticals. Indeed, organic cation transporter (OCT)2 is the initial step in the renal secretion of organic cations and consequently plays a defining role in establishing the pharmacokinetics of many cationic drugs. Although a hallmark of OCTs is their broad selectivity, this characteristic also makes them targets for unwanted, adverse drug-drug interactions (DDIs), making them a focus for efforts to develop models of ligand interaction that could predict and preempt these adverse interactions. This review discusses the molecular characteristics of these transporters as well as the evidence that established the OCTs as key players in the distribution of organic cations. However, the primary focus is the present understanding of the complexity of ligand interaction with OCTs, particularly OCT2, including evidence for the presence of multiple ligand-binding sites and the influence of substrate structure on the affinity of the transporter for inhibitory ligands. This leads to a discussion of the complexities associated with the development of protocols for assessing the inhibitory potential of new molecular entities to perpetrate unwanted DDIs, the criteria that should be considered in the interpretation of the results of such protocols, and the challenges associated with development of models capable of predicting unwanted DDIs.
Keywords: kidney; kinetics; organic cation; transport.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures

References
-
- Arndt P, Volk C, Gorboulev V, Budiman T, Popp C, Ulzheimer-Teuber I, Akhoundova A, Koppatz S, Bamberg E, Nagel G, Koepsell H. Interaction of cations, anions, and weak base quinine with rat renal cation transporter rOCT2 compared with rOCT1. Am J Physiol Renal Physiol 281: F454–F468, 2001. doi:10.1152/ajprenal.2001.281.3.F454. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

