Protective effect of Platymiscium floribundum Vog. in tree extract on periodontitis inflammation in rats
- PMID: 31682614
- PMCID: PMC6827912
- DOI: 10.1371/journal.pone.0223800
Protective effect of Platymiscium floribundum Vog. in tree extract on periodontitis inflammation in rats
Abstract
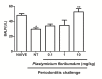
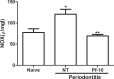
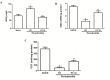
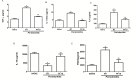
Periodontitis is an immuno-inflammatory disease, which can lead to tooth loss. This study aimed to investigate the efficacy of Platymiscium floribundum Vog., a Brazilian tree which has been used in folk medicine as an anti-inflammatory agent, in a pre-clinical trial of periodontitis in rats. Periodontitis was induced by placing a sterilized nylon (3.0) thread ligature around the cervix of the second left upper molar of the rats, which received (per os) P. floribundum extract (0.1, 1 or 10 mg/kg) or vehicle 1h before periodontitis-challenge and once daily during 11 days. Treatment with P. floribundum (10mg/kg) decreased alveolar bone loss, MPO activity nitrite/nitrate levels, oxidative stress, TNF-α, IL1-β, IL-8/CINC-1, and PGE2 gingival levels, and transcription of TNF-α, IL1-β, COX-2, iNOS, RANK, and RANKL genes, while elevated both BALP serum levels and IL-10 gingival levels. The animals did not show signs of toxicity throughout the experimental course. These findings show that P. floribundum has anti-inflammatory and anti-resorptive properties in a pre-clinical trial of periodontitis, representing an interesting biotechnological tool.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

