Antidepressant Tolerability in Pediatric Anxiety and Obsessive-Compulsive Disorders: A Bayesian Hierarchical Modeling Meta-analysis
- PMID: 31682918
- PMCID: PMC8028746
- DOI: 10.1016/j.jaac.2019.10.013
Antidepressant Tolerability in Pediatric Anxiety and Obsessive-Compulsive Disorders: A Bayesian Hierarchical Modeling Meta-analysis
Abstract
Objective: To compare adverse events (AEs), suicidality, and AE-related discontinuation in double-blind, placebo-controlled trials of pediatric patients with obsessive-compulsive disorder (OCD) and anxiety disorders treated with selective serotonin reuptake inhibitors (SSRIs) or serotonin-norepinephrine reuptake inhibitors (SNRIs).
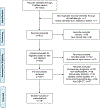
Method: MEDLINE, PubMed, Web of Science, PsycINFO, and Embase were searched for peer-reviewed, English-language articles from inception through March 1, 2019. We identified prospective, randomized SSRI and SNRI studies in patients <18 years of age with OCD or generalized, separation, or social anxiety disorders. AE rates were extracted and medication-placebo differences were examined using Bayesian hierarchical models, then posterior estimates of relative risk (RR) were determined for each AE by medication class and disorder.
Results: Data were included from 18 trials (2,631 patients) and 7 medications (16 SSRI and 4 SNRI trials). Compared with placebo, SSRIs were associated with a greater likelihood of AE-related discontinuation (RR 3.59, credible interval [CrI] 0.019-0.067, p = .0003), activation (RR 2.39, CrI 0.048-0.125, p = .003), sedation (RR 1.94, CrI 0.035-0.157, p = .002), insomnia (RR 1.93, CrI 0.040-0.149, p = .001), abdominal pain (RR 1.53, CrI 0.032-0.164, p = .005), and headache (RR 1.24, CrI 0.003-0.139, p = .04). Activation was more common with SSRIs (versus SNRIs, RR 1.32, CrI 0.018-0.114, p = .007). Neither SSRIs nor SNRIs were associated with treatment-emergent suicidality.
Conclusion: In pediatric OCD and anxiety disorders, SSRIs (compared with placebo) are associated with distinct AEs and greater AE-related discontinuation, although their tolerability does not differ between anxiety disorders and OCD. Compared with SNRIs, SSRIs are more likely to produce activation. Class-related AEs are important for clinicians to consider, particularly in light of data suggesting differences in class-related efficacy. Whereas SSRIs are superior to SNRIs and the treatment of choice for anxiety, for youths who become activated on SSRIs, SNRIs might represent a good second choice given their reported efficacy and lower risk of activation.
Keywords: OCD; SNRI; SSRI; antidepressant; anxiety.
Copyright © 2019 American Academy of Child and Adolescent Psychiatry. Published by Elsevier Inc. All rights reserved.
Figures


Similar articles
-
The Impact of Development on Antidepressant and Placebo Response in Anxiety Disorders: A Bayesian Hierarchical Meta-Analytic Examination of Randomized Controlled Trials in Children, Adolescents, and Adults.J Child Adolesc Psychopharmacol. 2024 Sep;34(7):302-309. doi: 10.1089/cap.2024.0016. Epub 2024 May 27. J Child Adolesc Psychopharmacol. 2024. PMID: 38800869
-
Efficacy and Safety of Selective Serotonin Reuptake Inhibitors, Serotonin-Norepinephrine Reuptake Inhibitors, and Placebo for Common Psychiatric Disorders Among Children and Adolescents: A Systematic Review and Meta-analysis.JAMA Psychiatry. 2017 Oct 1;74(10):1011-1020. doi: 10.1001/jamapsychiatry.2017.2432. JAMA Psychiatry. 2017. PMID: 28854296 Free PMC article.
-
Trajectory and magnitude of response in adults with anxiety disorders: a Bayesian hierarchical modeling meta-analysis of selective serotonin reuptake inhibitors, serotonin norepinephrine reuptake inhibitors, and benzodiazepines.CNS Spectr. 2024 Jun;29(3):187-196. doi: 10.1017/S1092852924000142. Epub 2024 Mar 25. CNS Spectr. 2024. PMID: 38523533
-
The Impact of Antidepressant Dose and Class on Treatment Response in Pediatric Anxiety Disorders: A Meta-Analysis.J Am Acad Child Adolesc Psychiatry. 2018 Apr;57(4):235-244.e2. doi: 10.1016/j.jaac.2018.01.015. Epub 2018 Feb 8. J Am Acad Child Adolesc Psychiatry. 2018. PMID: 29588049 Free PMC article.
-
Adverse Effects of Antidepressant Medications and their Management in Children and Adolescents.Pharmacotherapy. 2023 Jul;43(7):675-690. doi: 10.1002/phar.2767. Epub 2023 Jan 27. Pharmacotherapy. 2023. PMID: 36651686 Free PMC article. Review.
Cited by
-
The Impact of Failed Antidepressant Trials on Outcomes in Children and Adolescents with Anxiety and Depression: A Systematic Review and Meta-Analysis.J Child Adolesc Psychopharmacol. 2021 May;31(4):259-267. doi: 10.1089/cap.2020.0195. Epub 2021 Apr 21. J Child Adolesc Psychopharmacol. 2021. PMID: 33887154 Free PMC article.
-
Computational Predictions for OCD Pathophysiology and Treatment: A Review.Front Psychiatry. 2021 Oct 1;12:687062. doi: 10.3389/fpsyt.2021.687062. eCollection 2021. Front Psychiatry. 2021. PMID: 34658945 Free PMC article. Review.
-
Letter to the Editor: Sleep Disturbances in Selective Serotonin Reuptake Inhibitor-Treated Youth with Anxiety Disorders and Obsessive Compulsive Disorder-A Bayesian Hierarchical Modeling Meta-Analysis.J Child Adolesc Psychopharmacol. 2021 Jun;31(5):387-388. doi: 10.1089/cap.2020.0169. Epub 2021 Feb 10. J Child Adolesc Psychopharmacol. 2021. PMID: 33571033 Free PMC article. No abstract available.
-
Assessing access: Texting hotline app provides mental health crisis care for economically deprived youth.Soc Sci Med. 2024 Nov;361:117369. doi: 10.1016/j.socscimed.2024.117369. Epub 2024 Sep 26. Soc Sci Med. 2024. PMID: 39369499
-
Cognitive Behavioral Therapy for Anxiety Disorders in Youth: Efficacy, Moderators, and New Advances in Predicting Outcomes.Curr Psychiatry Rep. 2022 Dec;24(12):853-859. doi: 10.1007/s11920-022-01384-7. Epub 2022 Nov 12. Curr Psychiatry Rep. 2022. PMID: 36370264 Free PMC article. Review.
References
-
- The Research Unit on Pediatric Psychopharmacology Anxiety Study Group. Fluvoxamine for the treatment of anxiety disorders in children and adolescents. N Engl J Med. 2001;344(17):1279–1285. - PubMed
-
- Pediatric OCD Treatment Study (POTS) Team. Cognitive-behavior therapy, sertraline, and their combination for children and adolescents with obsessive-compulsive disorder: the Pediatric OCD Treatment Study (POTS) randomized controlled trial. JAMA. 2004;292(16):1969–1976. - PubMed
-
- Alderman J, Wolkow R, Chung M, Johnston HF. Sertraline treatment of children and adolescents with obsessive-compulsive disorder or depression: pharmacokinetics, tolerability, and efficacy. J Am Acad Child Adolesc Psychiatry. 1998;37(4):386–394. - PubMed

