D-Stellate Neurons of the Ventral Cochlear Nucleus Decrease in Auditory Nerve-Evoked Activity during Age-Related Hearing Loss
- PMID: 31683609
- PMCID: PMC6896102
- DOI: 10.3390/brainsci9110302
D-Stellate Neurons of the Ventral Cochlear Nucleus Decrease in Auditory Nerve-Evoked Activity during Age-Related Hearing Loss
Abstract
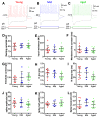
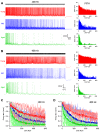
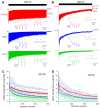
Age-related hearing loss (ARHL) is associated with weakened inhibition in the central auditory nervous system including the cochlear nucleus. One of the main inhibitory neurons of the cochlear nucleus is the D-stellate neuron, which provides extensive glycinergic inhibition within the local neural network. It remains unclear how physiological activities of D-stellate neurons change during ARHL and what are the underlying mechanisms. Using in vitro whole-cell patch clamp technique, we studied the intrinsic membrane properties of D-stellate neurons, the changes of their firing properties, and the underlying mechanisms in CBA/CaJ mice at the ages of 3-4 months (young), 17-19 months (middle age), and 27-33 months (aged). We found that the intrinsic membrane properties of D-stellate neurons were unchanged among these three age groups. However, these neurons showed decreased firing rate with age in response to sustained auditory nerve stimulation. Further investigation showed that auditory nerve-evoked excitatory postsynaptic currents (EPSCs) were significantly reduced in strength with age. These findings suggest that D-stellate neurons receive weakened synaptic inputs from the auditory nerve and decreased sound driven activity with age, which are expected to reduce the overall inhibition and enhance the central gain in the cochlear nucleus during ARHL.
Keywords: D-stellate; EPSC; age-related hearing loss; auditory nerve; firing rate; intrinsic property; synaptic transmission.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources

