A Chemometric Analysis of Deep-Sea Natural Products
- PMID: 31683674
- PMCID: PMC6865307
- DOI: 10.3390/molecules24213942
A Chemometric Analysis of Deep-Sea Natural Products
Abstract
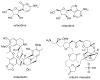
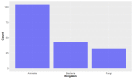
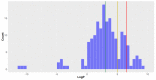
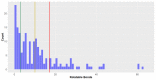
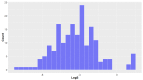
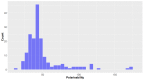
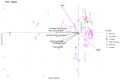
Deep-sea natural products have been created by unique marine organisms that thrive in a challenging environment of extreme conditions for its inhabitants. In this study, 179 deep-sea natural products isolated from 2009 to 2013 were investigated by analysing their physicochemical properties that are important indicators of the ADMET (Absorption, Distribution, Metabolism, Excretion and Toxicity) profile of a compound. The study and analysis of these molecular descriptors and characteristics enabled the defining of these compounds in various chemical spaces, particularly as an indication of their drug-likeness and position in chemical space and is the first to be conducted to analyse deep-sea derived natural products. It was found that ~40% of all deep-sea natural products were drug-like and 2/3 were within Known Drug Space (KDS), highlighting the high drug-likeness of a significant proportion of deep-sea natural products, most of which have already been shown to have notable biological activities, that should be further investigated as potential therapeutics. Furthermore, this study was able to reveal the general structural differences between compounds from Animalia, Bacteria and Fungi organisms where it was observed that natural products from members of the Animalia kingdom are structurally more varied than compounds from bacteria and fungi. It was also noted that, in general, fungi-derived compounds occupy a more favourable position in drug-like chemical space and are a rich and promising source of biologically-active natural products for the purposes of drug development and therapeutic application.
Keywords: chemical space; deep-sea; drug-like; known drug space; lead-like; natural products.
Conflict of interest statement
The author declares no conflict of interest.
Figures
















References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

