Classification of WHO Essential Oral Medicines for Children Applying a Provisional Pediatric Biopharmaceutics Classification System
- PMID: 31683740
- PMCID: PMC6920833
- DOI: 10.3390/pharmaceutics11110567
Classification of WHO Essential Oral Medicines for Children Applying a Provisional Pediatric Biopharmaceutics Classification System
Abstract
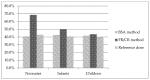
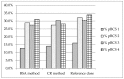
The objective was using the Essential Medicines List for children by the World Health Organization (WHO) to create a pediatric biopharmaceutics classification system (pBCS) of the oral drugs included in the Essential Medicines List by the World Health Organization and to compare our results with the BCS for adults (aBCS). Several methods to estimate the oral drug dose in different pediatric groups were used to calculate dose number (Do) and solubility (high/low). The estimation of the gastrointestinal water volume was adapted to each pediatric group. Provisional permeability classification was done by comparison of each drug lipophilicity versus metoprolol as the model drug of high permeability. As a result, 24.5% of the included drugs moved from the favorable to unfavorable class (i.e., from high to low solubility). Observed changes point out potential differences in product performance in pediatrics compared to adults, due to changes in the limiting factors for absorption. BCS Class Changes 1 to 2 or 3 to 4 are indicative of drugs that could be more sensitive to the choice of appropriate excipient in the development process. Validating a pBCS for each age group would provide a valuable tool to apply in specific pediatric formulation design by reducing time and costs and avoiding unnecessary pediatric experiments restricted due to ethical reasons. Additionally, pBCS could minimize the associated risks to the use of adult medicines or pharmaceutical compound formulations.
Keywords: BCS; biopharmaceutics; pediatrics; permeability; security; solubility.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- WHO WHO Model Lists of Essential Medicines. [(accessed on 19 June 2017)]; Available online: http://www.who.int/medicines/publications/essentialmedicines/en/
LinkOut - more resources
Full Text Sources
Research Materials

