Translation from the Ribosome to the Clinic: Implication in Neurological Disorders and New Perspectives from Recent Advances
- PMID: 31683805
- PMCID: PMC6920867
- DOI: 10.3390/biom9110680
Translation from the Ribosome to the Clinic: Implication in Neurological Disorders and New Perspectives from Recent Advances
Abstract
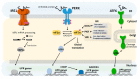
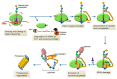
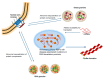
De novo protein synthesis by the ribosome and its multitude of co-factors must occur in a tightly regulated manner to ensure that the correct proteins are produced accurately at the right time and, in some cases, also in the proper location. With novel techniques such as ribosome profiling and cryogenic electron microscopy, our understanding of this basic biological process is better than ever and continues to grow. Concurrently, increasing attention is focused on how translational regulation in the brain may be disrupted during the progression of various neurological disorders. In fact, translational dysregulation is now recognized as the de facto pathogenic cause for some disorders. Novel mechanisms including ribosome stalling, ribosome-associated quality control, and liquid-liquid phase separation are closely linked to translational regulation, and may thus be involved in the pathogenic process. The relationships between translational dysregulation and neurological disorders, as well as the ways through which we may be able to reverse those detrimental effects, will be examined in this review.
Keywords: mRNA translational regulation; neurological disorders; phase separation; ribosome stalling; ribosome-associated quality control; tRNA dynamics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Gkogkas C.G., Khoutorsky A., Cao R., Jafarnejad S.M., Prager-Khoutorsky M., Giannakas N., Kaminari A., Fragkouli A., Nader K., Price T.J., et al. Pharmacogenetic Inhibition of eIF4E-Dependent Mmp9 mRNA Translation Reverses Fragile X Syndrome-like Phenotypes. Cell Rep. 2014;9:1742–1755. doi: 10.1016/j.celrep.2014.10.064. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

