NtMYB3, an R2R3-MYB from Narcissus, Regulates Flavonoid Biosynthesis
- PMID: 31683873
- PMCID: PMC6862390
- DOI: 10.3390/ijms20215456
NtMYB3, an R2R3-MYB from Narcissus, Regulates Flavonoid Biosynthesis
Abstract
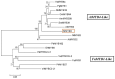
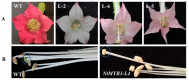
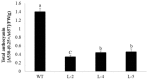
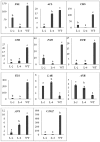
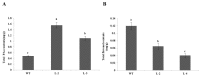
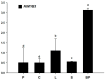
R2R3-MYB transcription factors play important roles in the regulation of plant flavonoid metabolites. In the current study, NtMYB3, a novel R2R3-MYB transcriptional factor isolated from Chinese narcissus (Narcissus tazetta L. var. chinensis), was functionally characterized. Phylogenetic analysis indicated that NtMYB3 belongs to the AtMYB4-like clade, which includes repressor MYBs involved in the regulation of flavonoid biosynthesis. Transient assays showed that NtMYB3 significantly reduced red pigmentation induced by the potato anthocyanin activator StMYB-AN1 in agro-infiltrated leaves of tobacco. Over-expression of NtMYB3 decreased the red color of transgenic tobacco flowers, with qRT-PCR analysis showing that NtMYB3 repressed the expression levels of genes involved in anthocyanin and flavonol biosynthesis. However, the proanthocyanin content in flowers of transgenic tobacco increased as compared to wild type. NtMYB3 showed expression in all examined narcissus tissues; the expression level in basal plates of the bulb was highest. A 968 bp promoter fragment of narcissus FLS (NtFLS) was cloned, and transient expression and dual luciferase assays showed NtMYB3 repressed the promoter activity. These results reveal that NtMYB3 is involved in the regulation of flavonoid biosynthesis in narcissus by repressing the biosynthesis of flavonols, and this leads to proanthocyanin accumulation in the basal plate of narcissus.
Keywords: Chinese narcissus; R2R3-MYB; anthocyanin; flavonoid repressor; flavonol.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Wu J., Chen L., Gu L., Wang Y., Tian H. Embryological studies on narcissus tazetta var. chinensis. J. Xiamen Univ. Nat. Sci. 2004;44:112–115.
-
- Winkel, Brenda S.J. Sci. Flavonoids. Springer; New York, NY, USA: 2006. The biosynthesis of flavonoids; pp. 71–95.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

