In Vivo Autophagy Up-Regulation of Small Intestine Enterocytes in Chinese Soft-Shelled Turtles during Hibernation
- PMID: 31683886
- PMCID: PMC6920937
- DOI: 10.3390/biom9110682
In Vivo Autophagy Up-Regulation of Small Intestine Enterocytes in Chinese Soft-Shelled Turtles during Hibernation
Abstract
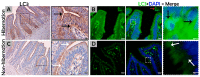
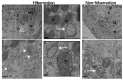
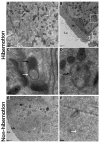
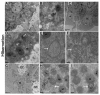
Many studies have focused on how autophagy plays an important role in intestinal homeostasis under pathological conditions. However, its role in the intestine during hibernation remains unclear. In the current study, we characterized in vivo up-regulation of autophagy in enterocytes of the small intestine of Chinese soft-shelled turtles during hibernation. Autophagy-specific markers were used to confirm the existence of autophagy in enterocytes through immunohistochemistry (IHC), immunofluorescence (IF), and immunoblotting. IHC staining indicated strong, positive immunoreactivity of the autophagy-related gene (ATG7), microtubule-associated protein light chain (LC3), and lysosomal-associated membrane protein 1 (LAMP1) within the mucosal surface during hibernation and poor expression during nonhibernation. IF staining results showed the opposite tendency for ATG7, LC3, and sequestosome 1 (p62). During hibernation ATG7 and LC3 showed strong, positive immunosignaling within the mucosal surface, while p62 showed strong, positive immunosignaling during nonhibernation. Similar findings were confirmed by immunoblotting. Moreover, the ultrastructural components of autophagy in enterocytes were revealed by transmission electron microscopy (TEM). During hibernation, the cumulative formation of phagophores and autophagosomes were closely associated with well-developed rough endoplasmic reticulum in enterocytes. These autophagosomes overlapped with lysosomes, multivesicular bodies, and degraded mitochondria to facilitate the formation of autophagolysosome, amphisomes, and mitophagy in enterocytes. Immunoblotting showed the expression level of PTEN-induced kinase 1 (PINK1), and adenosine monophosphate-activated protein kinase (AMPK) was enhanced during hibernation. Furthermore, the exosome secretion pathway of early-late endosomes and multivesicular bodies were closely linked with autophagosomes in enterocytes during hibernation. These findings suggest that the entrance into hibernation is a main challenge for reptiles to maintain homeostasis and cellular quality control in the intestine.
Keywords: ATG7; Chinese soft-shelled turtle; LC3; autophagosome; autophagy; enterocytes; hibernation; p62.
Conflict of interest statement
No potential conflicts of interest in this work.
Figures




Similar articles
-
LIPOPHAGY: a novel form of steroidogenic activity within the LEYDIG cell during the reproductive cycle of turtle.Reprod Biol Endocrinol. 2019 Feb 9;17(1):19. doi: 10.1186/s12958-019-0462-2. Reprod Biol Endocrinol. 2019. PMID: 30738428 Free PMC article.
-
Seasonal exploration of ultrastructure and Na+/K+-ATPase, Na+/K+/2Cl- cotransporter of mitochondria-rich cells in the small intestine of turtles.Micron. 2019 Nov;126:102747. doi: 10.1016/j.micron.2019.102747. Epub 2019 Aug 29. Micron. 2019. PMID: 31505373
-
Effect of seasonal variance on intestinal epithelial barriers and the associated innate immune response of the small intestine of the Chinese soft-shelled turtles.Fish Shellfish Immunol. 2020 Feb;97:173-181. doi: 10.1016/j.fsi.2019.12.042. Epub 2019 Dec 17. Fish Shellfish Immunol. 2020. PMID: 31857223
-
In Vivo Multivesicular Body and Exosome Secretion in the Intestinal Epithelial Cells of Turtles During Hibernation.Microsc Microanal. 2019 Dec;25(6):1341-1351. doi: 10.1017/S1431927619015071. Microsc Microanal. 2019. PMID: 31656212
-
The role of autophagy in ischemic brain injury.Autophagy Rep. 2025 Apr 3;4(1):2486445. doi: 10.1080/27694127.2025.2486445. eCollection 2025. Autophagy Rep. 2025. PMID: 40395988 Free PMC article. Review.
Cited by
-
Mechanisms of Cadmium-Induced Testicular Injury: A Risk to Male Fertility.Cells. 2022 Nov 14;11(22):3601. doi: 10.3390/cells11223601. Cells. 2022. PMID: 36429028 Free PMC article. Review.
-
Pathological Roles of Mitochondrial Oxidative Stress and Mitochondrial Dynamics in Cardiac Microvascular Ischemia/Reperfusion Injury.Biomolecules. 2020 Jan 5;10(1):85. doi: 10.3390/biom10010085. Biomolecules. 2020. PMID: 31948043 Free PMC article. Review.
-
Cross-Talk Between Selenium Nanoparticles and Cancer Treatment Through Autophagy.Biol Trace Elem Res. 2024 Jul;202(7):2931-2940. doi: 10.1007/s12011-023-03886-8. Epub 2023 Oct 10. Biol Trace Elem Res. 2024. PMID: 37817045 Review.
-
Integrated analysis of transcriptome and metabolome data reveals insights for molecular mechanisms in overwintering Tibetan frogs, Nanorana parkeri.Front Physiol. 2023 Jan 9;13:1104476. doi: 10.3389/fphys.2022.1104476. eCollection 2022. Front Physiol. 2023. PMID: 36699683 Free PMC article.
References
-
- Pan M. Hibernation induction in non-hibernating species. Biosci. Horiz. Int. J. Stud. Res. 2018;11 doi: 10.1093/biohorizons/hzy002. - DOI
-
- Vistro W.A., Tarique I., Haseeb A., Yang P., Huang Y., Chen H., Bai X., Fazlani S.A., Chen Q. Seasonal exploration of ultrastructure and Na+/K+-ATPase, Na+/K+/2Cl–cotransporter of mitochondria-rich cells in the small intestine of turtles. Micron. 2019:102747. doi: 10.1016/j.micron.2019.102747. - DOI - PubMed
-
- Hume I., Beiglböck C., Ruf T., Frey-Roos F., Bruns U., Arnold W. Seasonal changes in morphology and function of the gastrointestinal tract of free-living Alpine Marmots (Marmota marmota) J. Comp. Physiol. B. 2002;172:197–207. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

