Short-Term Therapies for Treatment of Acute and Advanced Heart Failure-Why so Few Drugs Available in Clinical Use, Why Even Fewer in the Pipeline?
- PMID: 31683969
- PMCID: PMC6912236
- DOI: 10.3390/jcm8111834
Short-Term Therapies for Treatment of Acute and Advanced Heart Failure-Why so Few Drugs Available in Clinical Use, Why Even Fewer in the Pipeline?
Abstract
Both acute and advanced heart failure are an increasing threat in term of survival, quality of life and socio-economical burdens. Paradoxically, the use of successful treatments for chronic heart failure can prolong life but-per definition-causes the rise in age of patients experiencing acute decompensations, since nothing at the moment helps avoiding an acute or final stage in the elderly population. To complicate the picture, acute heart failure syndromes are a collection of symptoms, signs and markers, with different aetiologies and different courses, also due to overlapping morbidities and to the plethora of chronic medications. The palette of cardio- and vasoactive drugs used in the hospitalization phase to stabilize the patient's hemodynamic is scarce and even scarcer is the evidence for the agents commonly used in the practice (e.g. catecholamines). The pipeline in this field is poor and the clinical development chronically unsuccessful. Recent set backs in expected clinical trials for new agents in acute heart failure (AHF) (omecamtiv, serelaxine, ularitide) left a field desolately empty, where only few drugs have been approved for clinical use, for example, levosimendan and nesiritide. In this consensus opinion paper, experts from 26 European countries (Austria, Belgium, Croatia, Cyprus, Czech Republic, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Israel, Italy, The Netherlands, Norway, Poland, Portugal, Russia, Slovenia, Spain, Sweden, Switzerland, Turkey, U.K. and Ukraine) analyse the situation in details also by help of artificial intelligence applied to bibliographic searches, try to distil some lesson-learned to avoid that future projects would make the same mistakes as in the past and recommend how to lead a successful development project in this field in dire need of new agents.
Keywords: acute heart failure; advanced heart failure; clinical development; levosimendan; regulatory clinical trials; short-term hemodynamic therapy.
Conflict of interest statement
The authors declare no conflict of interest. P.P. is full time employee of Orion Pharma, where levosimendan, one of the NCEs described in the text, was discovered and developed. In the latest 5 years, the other authors have received grants and speaker honoraria by Orion Pharma for investigator-initiated studies and educational lectures, respectively.
Figures
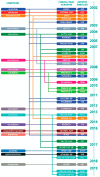
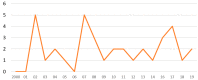

References
-
- Hamo C.E., Butler J., Gheorghiade M., Chioncel O. The bumpy road to drug development for acute heart failure. Eur. Heart J. Suppl. 2016;18:G19–G32. doi: 10.1093/eurheartj/suw045. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

