Measles Encephalitis: Towards New Therapeutics
- PMID: 31684034
- PMCID: PMC6893791
- DOI: 10.3390/v11111017
Measles Encephalitis: Towards New Therapeutics
Abstract
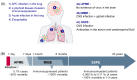
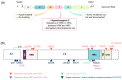
Measles remains a major cause of morbidity and mortality worldwide among vaccine preventable diseases. Recent decline in vaccination coverage resulted in re-emergence of measles outbreaks. Measles virus (MeV) infection causes an acute systemic disease, associated in certain cases with central nervous system (CNS) infection leading to lethal neurological disease. Early following MeV infection some patients develop acute post-infectious measles encephalitis (APME), which is not associated with direct infection of the brain. MeV can also infect the CNS and cause sub-acute sclerosing panencephalitis (SSPE) in immunocompetent people or measles inclusion-body encephalitis (MIBE) in immunocompromised patients. To date, cellular and molecular mechanisms governing CNS invasion are still poorly understood. Moreover, the known MeV entry receptors are not expressed in the CNS and how MeV enters and spreads in the brain is not fully understood. Different antiviral treatments have been tested and validated in vitro, ex vivo and in vivo, mainly in small animal models. Most treatments have high efficacy at preventing infection but their effectiveness after CNS manifestations remains to be evaluated. This review describes MeV neural infection and current most advanced therapeutic approaches potentially applicable to treat MeV CNS infection.
Keywords: central nervous system; measles virus; treatments; tropism.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

