Wnt Signaling in the Regulation of Immune Cell and Cancer Therapeutics
- PMID: 31684152
- PMCID: PMC6912555
- DOI: 10.3390/cells8111380
Wnt Signaling in the Regulation of Immune Cell and Cancer Therapeutics
Abstract
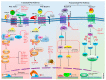
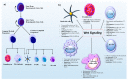
Wnt signaling is one of the important pathways to play a major role in various biological processes, such as embryonic stem-cell development, tissue regeneration, cell differentiation, and immune cell regulation. Recent studies suggest that Wnt signaling performs an essential function in immune cell modulation and counteracts various disorders. Nonetheless, the emerging role and mechanism of action of this signaling cascade in immune cell regulation, as well as its involvement in various cancers, remain debatable. The Wnt signaling in immune cells is very diverse, e.g., the tolerogenic role of dendritic cells, the development of natural killer cells, thymopoiesis of T cells, B-cell-driven initiation of T-cells, and macrophage actions in tissue repair, regeneration, and fibrosis. The purpose of this review is to highlight the current therapeutic targets in (and the prospects of) Wnt signaling, as well as the potential suitability of available modulators for the development of cancer immunotherapies. Although there are several Wnt inhibitors relevant to cancer, it would be worthwhile to extend this approach to immune cells.
Keywords: Wnt signaling; cancer; immune cell regulation; inhibitor; therapeutic target.
Conflict of interest statement
The authors declare that there are no competing interests.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

