Vaccines for preventing rotavirus diarrhoea: vaccines in use
- PMID: 31684685
- PMCID: PMC6816010
- DOI: 10.1002/14651858.CD008521.pub5
Vaccines for preventing rotavirus diarrhoea: vaccines in use
Update in
-
Vaccines for preventing rotavirus diarrhoea: vaccines in use.Cochrane Database Syst Rev. 2021 Nov 17;11(11):CD008521. doi: 10.1002/14651858.CD008521.pub6. Cochrane Database Syst Rev. 2021. PMID: 34788488 Free PMC article.
Abstract
Background: Rotavirus results in more diarrhoea-related deaths in children under five years than any other single agent in countries with high childhood mortality. It is also a common cause of diarrhoea-related hospital admissions in countries with low childhood mortality. Rotavirus vaccines that have been prequalified by the World Health Organization (WHO) include a monovalent vaccine (RV1; Rotarix, GlaxoSmithKline), a pentavalent vaccine (RV5; RotaTeq, Merck), and, more recently, another monovalent vaccine (Rotavac, Bharat Biotech).
Objectives: To evaluate rotavirus vaccines prequalified by the WHO (RV1, RV5, and Rotavac) for their efficacy and safety in children.
Search methods: On 4 April 2018 we searched MEDLINE (via PubMed), the Cochrane Infectious Diseases Group Specialized Register, CENTRAL (published in the Cochrane Library), Embase, LILACS, and BIOSIS. We also searched the WHO ICTRP, ClinicalTrials.gov, clinical trial reports from manufacturers' websites, and reference lists of included studies and relevant systematic reviews.
Selection criteria: We selected randomized controlled trials (RCTs) in children comparing rotavirus vaccines prequalified for use by the WHO versus placebo or no intervention.
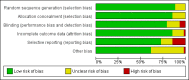
Data collection and analysis: Two review authors independently assessed trial eligibility and assessed risks of bias. One review author extracted data and a second author cross-checked them. We combined dichotomous data using the risk ratio (RR) and 95% confidence interval (CI). We stratified the analysis by country mortality rate and used GRADE to evaluate evidence certainty.
Main results: Fifty-five trials met the inclusion criteria and enrolled a total of 216,480 participants. Thirty-six trials (119,114 participants) assessed RV1, 15 trials (88,934 participants) RV5, and four trials (8432 participants) Rotavac. RV1 Children vaccinated and followed up the first year of life In low-mortality countries, RV1 prevents 84% of severe rotavirus diarrhoea cases (RR 0.16, 95% CI 0.09 to 0.26; 43,779 participants, 7 trials; high-certainty evidence), and probably prevents 41% of cases of severe all-cause diarrhoea (RR 0.59, 95% CI 0.47 to 0.74; 28,051 participants, 3 trials; moderate-certainty evidence). In high-mortality countries, RV1 prevents 63% of severe rotavirus diarrhoea cases (RR 0.37, 95% CI 0.23 to 0.60; 6114 participants, 3 trials; high-certainty evidence), and 27% of severe all-cause diarrhoea cases (RR 0.73, 95% CI 0.56 to 0.95; 5639 participants, 2 trials; high-certainty evidence). Children vaccinated and followed up for two years In low-mortality countries, RV1 prevents 82% of severe rotavirus diarrhoea cases (RR 0.18, 95% CI 0.14 to 0.23; 36,002 participants, 9 trials; high-certainty evidence), and probably prevents 37% of severe all-cause diarrhoea episodes (rate ratio 0.63, 95% CI 0.56 to 0.71; 39,091 participants, 2 trials; moderate-certainty evidence). In high-mortality countries RV1 probably prevents 35% of severe rotavirus diarrhoea cases (RR 0.65, 95% CI 0.51 to 0.83; 13,768 participants, 2 trials; high-certainty evidence), and 17% of severe all-cause diarrhoea cases (RR 0.83, 95% CI 0.72 to 0.96; 2764 participants, 1 trial; moderate-certainty evidence). No increased risk of serious adverse events (SAE) was detected (RR 0.88 95% CI 0.83 to 0.93; high-certainty evidence). There were 30 cases of intussusception reported in 53,032 children after RV1 vaccination and 28 cases in 44,214 children after placebo or no intervention (RR 0.70, 95% CI 0.46 to 1.05; low-certainty evidence). RV5 Children vaccinated and followed up the first year of life In low-mortality countries, RV5 probably prevents 92% of severe rotavirus diarrhoea cases (RR 0.08, 95% CI 0.03 to 0.22; 4132 participants, 5 trials; moderate-certainty evidence). We did not identify studies reporting on severe all-cause diarrhoea in low-mortality countries. In high-mortality countries, RV5 prevents 57% of severe rotavirus diarrhoea (RR 0.43, 95% CI 0.29 to 0.62; 5916 participants, 2 trials; high-certainty evidence), but there is probably little or no difference between vaccine and placebo for severe all-cause diarrhoea (RR 0.80, 95% CI 0.58 to 1.11; 1 trial, 4085 participants; moderate-certainty evidence). Children vaccinated and followed up for two years In low-mortality countries, RV5 prevents 82% of severe rotavirus diarrhoea cases (RR 0.18, 95% CI 0.08 to 0.39; 7318 participants, 4 trials; moderate-certainty evidence). We did not identify studies reporting on severe all-cause diarrhoea in low-mortality countries. In high-mortality countries, RV5 prevents 41% of severe rotavirus diarrhoea cases (RR 0.59, 95% CI 0.43 to 0.82; 5885 participants, 2 trials; high-certainty evidence), and 15% of severe all-cause diarrhoea cases (RR 0.85, 95% CI 0.75 to 0.98; 5977 participants, 2 trials; high-certainty evidence). No increased risk of serious adverse events (SAE) was detected (RR 0.93 95% CI 0.86 to 1.01; moderate to high-certainty evidence). There were 16 cases of intussusception in 43,629 children after RV5 vaccination and 20 cases in 41,866 children after placebo (RR 0.77, 95% CI 0.41 to 1.45; low-certainty evidence). Rotavac Children vaccinated and followed up the first year of life Rotavac has not been assessed in any RCT in countries with low child mortality. In India, a high-mortality country, Rotavac probably prevents 57% of severe rotavirus diarrhoea cases (RR 0.43, 95% CI 0.30 to 0.60; 6799 participants, moderate-certainty evidence); the trial did not report on severe all-cause diarrhoea at one-year follow-up. Children vaccinated and followed up for two years Rotavac probably prevents 54% of severe rotavirus diarrhoea cases in India (RR 0.46, 95% CI 0.35 to 0.60; 6541 participants, 1 trial; moderate-certainty evidence), and 16% of severe all-cause diarrhoea cases (RR 0.84, 95% CI 0.71 to 0.98; 6799 participants, 1 trial; moderate-certainty evidence). No increased risk of serious adverse events (SAE) was detected (RR 0.93 95% CI 0.85 to 1.02; moderate-certainty evidence). There were eight cases of intussusception in 5764 children after Rotavac vaccination and three cases in 2818 children after placebo (RR 1.33, 95% CI 0.35 to 5.02; very low-certainty evidence). There was insufficient evidence of an effect on mortality from any rotavirus vaccine (198,381 participants, 44 trials; low- to very low-certainty evidence), as the trials were not powered to detect an effect at this endpoint.
Authors' conclusions: RV1, RV5, and Rotavac prevent episodes of rotavirus diarrhoea. Whilst the relative effect estimate is smaller in high-mortality than in low-mortality countries, there is a greater number of episodes prevented in these settings as the baseline risk is much higher. We found no increased risk of serious adverse events. 21 October 2019 Up to date All studies incorporated from most recent search All published trials found in the last search (4 Apr, 2018) were included and 15 ongoing studies are currently awaiting completion (see 'Characteristics of ongoing studies').
Contexte: Le rotavirus entraîne plus de décès liés à la diarrhée chez les enfants de moins de cinq ans que tout autre agent unique dans les pays où la mortalité infantile est élevée. C'est également une cause fréquente d'hospitalisations liées à la diarrhée dans les pays où la mortalité infantile est faible. Les vaccins antirotavirus préqualifiés par l'Organisation mondiale de la santé (OMS) comprennent un vaccin monovalent (RV1 ; Rotarix, GlaxoSmithKline), un vaccin pentavalent (RV5 ; RotaTeq, Merck) et plus récemment un autre vaccin monovalent (Rotavac, Bharat Biotech).
Objectifs: Évaluer les vaccins antirotavirus préqualifiés par l'OMS (RV1, RV5 et Rotavac) pour leur efficacité et leur innocuité chez les enfants. STRATÉGIE DE RECHERCHE DOCUMENTAIRE: Le 4 avril 2018, nous avons effectué une recherche dans MEDLINE (via PubMed), le registre spécialisé du groupe de travail Cochrane sur les maladies infectieuses (the Cochrane Infectious Diseases Group), CENTRAL (publié dans la Bibliothèque Cochrane), Embase, LILACS, et BIOSIS. Nous avons également effectué des recherches dans le système d’enregistrement international des essais cliniques (ICTRP) de l'OMS, ClinicalTrials.gov, les rapports d'essais cliniques trouvés sur les sites Web des fabricants, les références des études incluses et les revues systématiques pertinentes. CRITÈRES DE SÉLECTION: Nous avons sélectionné des essais cliniques contrôlés randomisés (ECR) chez des enfants comparant des vaccins antirotavirus préqualifiés pour utilisation par l'OMS à un placebo ou à aucune intervention. RECUEIL ET ANALYSE DES DONNÉES: Deux auteurs de la revue ont évalué de façon indépendante l'éligibilité à l'essai et évalué les risques de biais. Un auteur de la revue a extrait les données et un deuxième auteur les a vérifiées par recoupement. Nous avons combiné des données dichotomiques en utilisant le risque relatif (RR) et l'intervalle de confiance à 95 % (IC). Nous avons stratifié l'analyse par taux de mortalité par pays et utilisé GRADE pour évaluer la valeur probante des données. RÉSULTATS PRINCIPAUX: Cinquante‐cinq essais ont satisfait aux critères d'inclusion et enrôlé 216 480 participants au total. Trente‐six essais cliniques (119 114 participants) ont évalué le RV1, 15 essais cliniques (88 934 participants) le RV5 et quatre essais cliniques (8432 participants) le Rotavac. RV1 Enfants vaccinés et suivis au cours de leur première année de vie Dans les pays à faible mortalité, le RV1 prévient 84 % des cas graves de diarrhée à rotavirus (RR 0,16, IC à 95 % : 0,09 à 0,26 ; 43 779 participants, 7 essais ; données de bonne valeur probante) et probablement 41 % des cas de diarrhée sévère toutes causes confondues (RR 0,59, IC à 95 % : 0,47 à 0,74 ; 28 051 participants, 3 essais ; données de valeur probante moyenne). Dans les pays à forte mortalité, le RV1 prévient 63 % des cas graves de diarrhée à rotavirus (RR 0,37, IC à 95 % : 0,23 à 0,60 ; 6114 participants, 3 essais ; données de bonne valeur probante) et 27 % des cas graves de diarrhée toutes causes confondues (RR 0,73, IC à 95 % : 0,56 à 0,95 ; 5639 participants, 2 essais ; données de bonne valeur probante). Enfants vaccinés et suivis pendant deux ans Dans les pays à faible mortalité, le RV1 prévient 82 % des cas graves de diarrhée à rotavirus (RR 0,18, IC à 95 % : 0,14 à 0,23 ; 36 002 participants, 9 essais ; données de bonne valeur probante) et probablement 37 % des épisodes graves de diarrhée toutes causes confondues (rapport des taux 0,63, IC à 95 % : 0,56 à 0,71 ; 39 091 participants, 2 essais ; données de valeur probante moyenne). Dans les pays à forte mortalité, le RV1 prévient probablement 35 % des cas graves de diarrhée à rotavirus (RR 0,65, IC à 95 % : 0,51 à 0,83 ; 13 768 participants, 2 essais ; données de bonne valeur probante) et 17 % des cas graves de diarrhée toutes causes confondues (RR 0,83, IC à 95 % : 0,72 à 0,96 ; 2764 participants, 1 essai ; données de valeur probante moyenne). Aucune augmentation du risque d'événements indésirables graves (EIG) n'a été décelée (RR 0,88 IC à 95 % 0,83 à 0,93 ; données de bonne valeur probante). On a signalé 30 cas d’invagination (intussusception) intestinale chez 53 032 enfants après la vaccination RV1 et 28 cas chez 44 214 enfants après l'administration d'un placebo ou l'absence d'intervention (RR 0,70, IC à 95 % : 0,46 à 1,05 ; données de faible valeur probante). RV5 Enfants vaccinés et suivis au cours de leur première année de vie Dans les pays à faible mortalité, le RV5 prévient probablement 92 % des cas graves de diarrhée à rotavirus (RR 0,08, IC à 95 % : 0,03 à 0,22 ; 4 132 participants, 5 essais ; données de valeur probante moyenne). Nous n'avons pas identifié d'études sur les diarrhées graves toutes causes confondues dans les pays à faible mortalité. Dans les pays à forte mortalité, le RV5 prévient 57 % des cas de diarrhée à rotavirus grave (RR 0,43, IC à 95 % : 0,29 à 0,62 ; 5916 participants, 2 essais ; données de bonne valeur probante), mais il n’y a probablement que peu voire pas de différence entre vaccin et placebo pour la diarrhée grave toutes causes confondues (RR 0,80, IC à 95 % : 0,58 à 1,11 ; 1 essai, 4085 participants ; données de valeur probante moyenne). Enfants vaccinés et suivis pendant deux ans Dans les pays à faible mortalité, le RV5 prévient 82 % des cas graves de diarrhée à rotavirus (RR 0,18, IC à 95 % : 0,08 à 0,39 ; 7318 participants, 4 essais ; données de valeur probante moyenne). Nous n'avons pas identifié d'études sur les diarrhées graves toutes causes confondues dans les pays à faible mortalité. Dans les pays à forte mortalité, le RV5 prévient 41 % des cas graves de diarrhée à rotavirus (RR 0,59, IC à 95 % : 0,43 à 0,82 ; 5 885 participants, 2 essais ; données de bonne valeur probante) et 15 % des cas graves de diarrhée toutes causes confondues (RR 0,85, IC à 95 % : 0,75 à 0,98 ; 5977 participants, 2 essais ; données de bonne valeur probante). Aucune augmentation du risque d'évènements indésirables graves (EIG) n'a été décelée (RR 0,93 IC à 95 % 0,86 à 1,01 ; données de valeur probante moyenne à bonne). Il y a eu 16 cas d'invagination chez 43 629 enfants après la vaccination RV5 et 20 cas chez 41 866 enfants après le placebo (RR 0,77, IC à 95 % : 0,41 à 1,45 ; données de faible valeur probante). Rotavac Enfants vaccinés et suivis au cours de leur première année de vie Le Rotavac n'a fait l'objet d'aucun ECR dans les pays à faible mortalité infantile. En Inde, pays à forte mortalité, le Rotavac prévient probablement 57 % des cas graves de diarrhée à rotavirus (RR 0,43, IC à 95 % : 0,30 à 0,60 ; 6799 participants, données de valeur probante moyenne) ; l'essai n'a pas fait état de diarrhée grave toutes causes confondues à un an de suivi. Enfants vaccinés et suivis pendant deux ans Le Rotavac prévient probablement 54 % des cas graves de diarrhée à rotavirus en Inde (RR 0,46, IC à 95 % : 0,35 à 0,60 ; 6541 participants, 1 essai ; données de valeur probante moyenne) et 16 % des cas graves de diarrhée toutes causes confondues (RR 0,84, IC à 95 % : 0,71 à 0,98 ; 6799 participants, 1 essai ; données de valeur probante moyenne). Aucune augmentation du risque d'évènements indésirables graves (EIG) n'a été décelée (RR 0,93 95 % IC 0,85 à 1,02 ; données de valeur probante moyenne). Il y a eu huit cas d'invagination intestinale chez 5 764 enfants après la vaccination par Rotavac et trois cas chez 2 818 enfants après le placebo (RR 1,33, IC à 95 % : 0,35 à 5,02 ; données de très faible valeur probante). Il n'y avait pas suffisamment de données probante indiquant un effet sur la mortalité attribuable à un vaccin antirotavirus (198 381 participants, 44 essais ; données de valeur probante faible à très faible), car les essais n'étaient pas assez puissants pour détecter un effet à ce paramètre.
Conclusions des auteurs: Les vaccins RV1, RV5 et Rotavac préviennent les épisodes de diarrhée à rotavirus. Bien que l'estimation de l'effet relatif soit plus faible dans les pays à forte mortalité que dans les pays à faible mortalité, le nombre d'épisodes évités est plus élevé dans ces pays car le risque de base est beaucoup plus élevé. Nous n'avons trouvé aucun risque accru d'événements indésirables graves.
Copyright © 2019 The Authors. Cochrane Database of Systematic Reviews published by John Wiley & Sons, Ltd. on behalf of The Cochrane Collaboration.
Conflict of interest statement
Hanna Bergman: received payment for work on this review from Cochrane Response, an evidence services unit operated by the Cochrane Collaboration. Cochrane Response was contracted by the WHO to produce a systematic review upon which a part of this review update is based (see ‘Sources of support').
Nigel Cunliffe: received research grant support and honoraria for participation in Data Safety Monitoring Boards from GlaxoSmithKline Biologicals.
Femi Pitan: none known.
Nicholas Henschke: received payment for work on this review from Cochrane Response, an evidence services unit operated by the Cochrane Collaboration. Cochrane Response was contracted by the WHO to produce a systematic review upon which a part of this review update is based (see ‘Sources of support').
Karla Soares‐Weiser: has received payment in the past (not for the current update) to conduct this review from the DFID UK via the Effective Health Care Research Programme Consortium (see ‘Sources of support').
Figures


Update of
-
Vaccines for preventing rotavirus diarrhoea: vaccines in use.Cochrane Database Syst Rev. 2019 Mar 25;3(3):CD008521. doi: 10.1002/14651858.CD008521.pub4. Cochrane Database Syst Rev. 2019. Update in: Cochrane Database Syst Rev. 2019 Oct 28;2019(10). doi: 10.1002/14651858.CD008521.pub5. PMID: 30912133 Free PMC article. Updated.
References
References to studies included in this review
RV1 Anh 2011‐PHL {published data only}
-
- Anh DD, Carlos CC, Thiem DV, Hutagalung Y, Gatchalian S, Bock HL, et al. Immunogenicity, reactogenicity and safety of the human rotavirus vaccine RIX4414 (Rotarix) oral suspension (liquid formulation) when co‐administered with expanded program on immunization (EPI) vaccines in Vietnam and the Philippines in 2006‐2007. Vaccine 2011;29(11):2029‐36. - PubMed
-
- GlaxoSmithKline[109216‐063]. A phase II, randomized, double‐blind, placebo‐controlled study to evaluate the immunogenicity, reactogenicity and safety of two doses of GlaxoSmithKline (GSK) Biologicals’ oral live attenuated human rotavirus (HRV) liquid vaccine, when given to healthy infants, in Philippines. www.gsk‐studyregister.com/study/3204 (accessed 12 December 2018).
-
- NCT00432380. Immunogenicity and safety of two doses of GlaxoSmithKline (GSK) Biologicals' oral live attenuated human rotavirus (HRV) liquid vaccine (GSK 357941A) in healthy infants. clinicaltrials.gov/show/NCT00432380 (first received 7 February 2007).
RV1 Anh 2011‐VNM {published data only}
-
- Anh DD, Carlos CC, Thiem DV, Hutagalung Y, Gatchalian S, Bock HL, et al. Immunogenicity, reactogenicity and safety of the human rotavirus vaccine RIX4414 (Rotarix) oral suspension (liquid formulation) when co‐administered with expanded program on immunization (EPI) vaccines in Vietnam and the Philippines in 2006‐2007. Vaccine 2011;29(11):2029‐36. - PubMed
-
- Anh DD, Thiem VD, Hutagalung Y, Bock HL, Suryakiran P, Delem A, et al. Immunogenicity, reactogenicity and safety of the oral live attenuated human rotavirus vaccine RIX4414 (Rotarix™) oral suspension (liquid formulation) in Vietnamese Infants. 13th International Congress on Infectious Diseases Abstracts, Poster Presentations. Kuala Lumpur, Malaysia; June 19–22, 2008.
-
- GlaxoSmithKline[105722‐051]. A phase II, randomized, double‐blind, placebo‐controlled study to evaluate the immunogenicity, reactogenicity and safety of two doses of GlaxoSmithKline (GSK) Biologicals’ oral live attenuated human rotavirus (HRV) liquid vaccine, when given to healthy infants, in Vietnam. www.gsk‐studyregister.com/study/3012 (accessed 12 December 2018).
-
- NCT00345956. A placebo‐controlled study to evaluate the immunogenicity, reactogenicity and safety of two doses of GSK Bio oral live attenuated human rotavirus (HRV) liquid vaccine, when given to healthy infants, in Vietnam. clinicaltrials.gov/show/NCT00345956 (first received 29 June 2006).
RV1 Bernstein 1998‐USA {published data only}
-
- Bernstein DI, Smith VE, Sherwood JR, Schiff GM, Sander DS, DeFeudis D, et al. Safety and immunogenicity of live, attenuated human rotavirus vaccine 89‐12. Vaccine 1998;16(4):381‐7. - PubMed
RV1 Bernstein 1999‐USA {published data only}
-
- Bernstein DI, Sack DA, Reisinger K, Rothstein E, Ward RL. Second‐year follow‐up evaluation of live, attenuated human rotavirus vaccine 89‐12 in healthy infants. Journal of Infectious Diseases 2002;186(10):1487‐9. - PubMed
-
- Bernstein DI, Sack DA, Rothstein E, Reisinger K, Smith VE, O'Sullivan D, et al. Efficacy of live, attenuated, human rotavirus vaccine 89‐12 in infants: a randomised placebo‐controlled trial. Lancet 1999;354(9175):287‐90. - PubMed
RV1 Colgate 2016‐BGD {published data only}
-
- Colgate ER, Haque R, Dickson DM, Carmolli MP, Mychaleckyj JC, Nayak U, et al. Delayed dosing of oral rotavirus vaccine demonstrates decreased risk of rotavirus gastroenteritis associated with serum zinc: a randomized controlled trial. Clinical Infectious Diseases 2016;63(5):634‐41. - PubMed
-
- Kirkpatrick BD, Colgate ER, Mychaleckyj JC, Haque R, Dickson DM, Carmolli MP, et al. The "Performance of Rotavirus and Oral Polio Vaccines in Developing Countries" (PROVIDE) study: description of methods of an interventional study designed to explore complex biologic problems. American Journal of Tropical Medicine and Hygiene 2015;92(4):744‐51. - PMC - PubMed
-
- Lee B, Diehl SA, Colgate ER, Dickson DM, Uddin MI, Sharmin S, et al. Lewis antigen and secretor status mediate susceptibility to p‐genotype specific rotavirus infections but do not affect rotavirus vaccine performance among infants in Bangladesh. American Journal of Tropical Medicine and Hygiene 2017;97:226.
-
- NCT01375647. Exploration of the biologic basis for underperformance of oral polio and rotavirus vaccines in Bangladesh PROVIDE. clinicaltrials.gov/show/NCT01375647 (first received 17 June 2011).
RV1 Dennehy 2005‐NA {published data only}
-
- Dennehy PH. A short report on the highlights of world‐wide development of RIX4414: a North American experience comparative evaluation of safety and immunogenicity of two dosages of an oral live attenuated human rotavirus vaccine (RIX4414) in infants in the United States and Canada. Vaccine 2006;24(18):3780‐1. - PubMed
-
- Dennehy PH, Brady RC, Halperin SA, Ward RL, Alvey JC, Fischer FH Jr, et al. Comparative evaluation of safety and immunogenicity of two dosages of an oral live attenuated human rotavirus vaccine. Pediatric Infectious Disease Journal 2005;24(6):481‐8. - PubMed
-
- GlaxoSmithKline[444563‐005]. A phase II, double‐blind, randomized, placebo‐controlled study of two doses of GlaxoSmithKline Biologicals’ live attenuated human rotavirus (HRV) vaccine at different virus concentrations (10 5.2 and 10 6.4 ffu) in healthy infants (approximately 2 months of age at first dose) following a 0, 2 month schedule and previously uninfected with human rotavirus. www.gsk‐studyregister.com/study/6783 (accessed 12 December 2018).
-
- NCT00729001. Study of two doses of GSK Biologicals' live attenuated HRV vaccine (two different formulations) in healthy infants. clinicaltrials.gov/ct2/show/NCT00729001 (accessed 6 August 2008).
RV1 GSK[021] 2007‐PAN {published data only}
-
- GlaxoSmithKline[444563‐021]. A phase II, double‐blind, randomized, placebo‐controlled clinical study to assess the immunogenicity and reactogenicity of three doses of a modified vaccine formulation versus GlaxoSmithKline (GSK) Biologicals’ live attenuated human rotavirus (HRV) vaccine when orally administered to healthy infants at 2, 4 and 6 months of age. www.gsk‐studyregister.com/study/6789 (accessed 12 December 2018).
RV1 GSK[033] 2007‐LA {published data only}
-
- GlaxoSmithKline[444563‐033]. A phase III, randomized, double‐blind and placebo‐controlled study to assess the clinical consistency of three production lots of GSK Biologicals’ HRV vaccine in terms of immunogenicity and safety when given to healthy infants at 2 and 4 months of age. www.gsk‐studyregister.com/study/6794 (accessed 12 December 2018).
-
- López P, Galan Herrera JF, Cervantes Y, Costa Clemens SA, Aguirre F, Yarzabal JP. Three consecutive production lots of the human monovalent RIX4414 G1P(8) rotavirus vaccine, Rotarix™ induce a consistent immune response in Latin American infants. [Poster]. 4th World Congress of The World Society for Pediatric Infectious Diseases; 2005 September 01‐04; Warsaw, Poland. 2005. [Not available for review]
-
- NCT00757770. Assessment of Clinical Consistency of Three Production Lots of GSK Biologicals' HRV Vaccine. clinicaltrials.gov/ct2/show/NCT00757770 (first received 23 September 2008).
RV1 GSK[041] 2007‐KOR {published data only}
-
- GlaxoSmithKline[103478‐041]. A phase IIIb, double‐blind, randomized, placebo‐controlled, multicentre study to assess the immunogenicity, safety and reactogenicity of 2 doses of GlaxoSmithKline (GSK) Biologicals’ oral live attenuated human rotavirus (HRV) vaccine in healthy infants (6‐12 weeks of age at first dose) previously uninfected with human rotavirus. www.gsk‐studyregister.com/study/2714 (accessed 12 December 2018).
-
- NCT00134732. Assess the immunogenicity, safety & reactogenicity of 2 doses of GSK Biologicals’ oral live attenuated human rotavirus (HRV) vaccine in healthy infants (6‐12 weeks of age at first dose) previously uninfected with human rotavirus. clinicaltrials.gov/ct2/show/record/NCT00134732 (first receiveded 25 August 2005).
RV1 GSK[101555] 2008‐PHL {published data only}
-
- GlaxoSmithKline[101555]. A phase II, double‐blind, randomized, placebo‐controlled study to compare the immunogenicity, reactogenicity and safety of 2 different formulations of GlaxoSmithKline (GSK) Biologicals’ live attenuated human rotavirus (HRV) vaccine given as a two‐dose primary vaccination in healthy infants previously uninfected with HRV. www.gsk‐studyregister.com/study/2632 (accessed 12 December 2018).
RV1 Kawamura 2011‐JPN {published data only}
-
- GlaxoSmithKline [107625 (Rota‐056)]. Efficacy, safety, reactogenicity and immunogenicity study of the lyophilised formulation of Rotarix vaccine in healthy Japanese infants. www.gsk‐studyregister.com/study/3131 (accessed 12 December 2018).
-
- Kawamura N, Tokoeda Y, Mori S, Ohshima M, Okahata H, Ueda D, et al. Efficacy of human rotavirus vaccine RIX4414 in Japanese infants from 2 weeks post dose 2 up to data lock point. 28th Meeting of European Society for Paediatric Infectious Diseases (ESPID); 2010 May 04‐08; Nice, France. 2010.
-
- Kawamura N, Tokoeda Y, Oshima M, Okahata H, Tsutsumi H, Doorn LJ, et al. Efficacy, safety and immunogenicity of RIX4414 in Japanese infants during the first two years of life. Vaccine 2011;29(37):6335‐41. - PubMed
-
- NCT00480324. Efficacy, safety, reactogenicity and immunogenicity study of the lyophilised formulation of Rotarix vaccine in healthy Japanese infants. clinicaltrials.gov/show/NCT00480324 (first received 17 November 2010).
RV1 Kerdpanich 2010‐THA {published data only}
-
- GlaxoSmithKline[103477‐039]. A phase IIIb, partially blind, randomized, placebo‐controlled study to asses the effect on immunogenicity of administration of vaccine without buffering agent and to assess heat stability in terms of immunogenicity, reactogenicity and safety of GlaxoSmithKline Biologicals’ oral live attenuated human rotavirus (HRV) vaccine following a 0, 2 month schedule, in healthy infants previously uninfected with human rotavirus. www.gsk‐studyregister.com/study/2713 (accessed 12 December 2018).
-
- Kerdpanich A, Chokephaibulkit K, Watanaveeradej V, Vanprapar N, Hutagalung Y, Han HH, et al. Exposure to elevated temperature of 37°C for 7 days does not affect immunogenicity and reactogenicity of RIX4414. [Poster]. dsRNA Virus Meeting; 2006 October 21‐26; Cape Town, South Africa. 2006. [Not available for review]
-
- Kerdpanich A, Chokephaibulkit K, Watanaveeradej V, Vanprapar N, Simasathien S, Phavichitr N, et al. Immunogenicity of a human rotavirus vaccine (RIX4414) after storage at 37 degrees C for seven days. Human Vaccine 2011;7(1):74‐80. - PubMed
-
- Kerdpanich A, Chokephaibulkit K, Watanaveeradej V, Vanprapar N, Simasathien S, Phavichitr N, et al. Immunogenicity of a live‐attenuated human rotavirus RIX4414 vaccine with or without buffering agent. Human Vaccines 2010;6(3):254‐62. - PubMed
-
- NCT00169455. Assess the effect on immunogenicity of administration of vaccine without buffering agent & assess heat stability in terms of immunogenicity, reactogenicity & safety of GSK Biologicals’ oral live attenuated human rotavirus (HRV) vaccine following a 0,2 m schedule, in healthy infants previously uninfected with human rotavirus. clinicaltrials.gov/show/NCT00169455 (first received 15 September 2005).
RV1 Kim 2012‐KOR {published data only}
-
- GlaxoSmithKline[112269‐068]. Immunogenicity, reactogenicity and safety study to evaluate two doses of the lyophilised formulation of the human rotavirus (HRV) vaccine when administered to healthy Korean infants previously uninfected with HRV. www.gsk‐studyregister.com/study/3589 (accessed 12 December 2018).
-
- Kim JS, Bae CW, Lee KY, Park MS, Choi YY, Kim KN, et al. Immunogenicity, reactogenicity and safety of a human rotavirus vaccine (RIX4414) in Korean infants: a randomized, double‐blind, placebo‐controlled, phase IV study. Human Vaccines and Immunotherapeutics 2012;8(6):806‐12. - PubMed
-
- NCT00969228. Study to Evaluate Immunogenicity, Reactogenicity and Safety of Rotarix™ Vaccine in Korean Infants. clinicaltrials.gov/ct2/show/NCT00969228 (first received 1 September 2009).
RV1 Li 2013a‐CHN {published data only}
-
- GlaxoSmithKline[113552‐073]. Reactogenicity and safety of a single dose of GlaxoSmithKline (GSK) Biologicals' human rotavirus (HRV) vaccine (444563) in healthy children. www.gsk‐studyregister.com/study/3957 (accessed 12 December 2018).
-
- Li RC, Li YP, Mo ZJ, Luo D, Huang T, Kong JL, et al. Reactogenicity and safety of a liquid human rotavirus vaccine (RIX4414) in healthy adults, children and infants in China: randomized, double‐blind, placebo‐controlled Phase I studies. Human Vaccines and Immunotherapeutics 2013;9(8):1638‐42. - PMC - PubMed
-
- NCT01086436. Study to evaluate the safety of Rotarix in Chinese children. clinicaltrials.gov/ct2/show/NCT01086436 (first received on 15 March 2010).
RV1 Li 2013b‐CHN {published data only}
-
- GlaxoSmithKline[113518‐074]. Reactogenicity and safety of two doses of GlaxoSmithKline (GSK) Biologicals’ oral live attenuated liquid human rotavirus (HRV) vaccine 444563, in healthy infants. www.gsk‐studyregister.com/study/3947 (accessed 12 December 2018).
-
- Li RC, Li YP, Mo ZJ, Luo D, Huang T, Kong JL, et al. Reactogenicity and safety of a liquid human rotavirus vaccine (RIX4414) in healthy adults, children and infants in China: randomized, double‐blind, placebo‐controlled Phase I studies. Human Vaccines and Immunotherapeutics 2013;9(8):1638‐42. - PMC - PubMed
-
- NCT01107587. Study to assess the safety of GSK Biologicals' liquid human rotavirus vaccine in healthy infants. clinicaltrials.gov/ct2/show/NCT01107587 (accessed 21 April 2010).
RV1 Li 2014‐CHN {published data only}
-
- GlaxoSmithKline[113808‐075]. Efficacy, immunogenicity and safety of two doses of GlaxoSmithKline (GSK) Biologicals' Oral Live Attenuated Liquid Human Rotavirus (HRV) Vaccine (444563), in healthy infants. www.gsk‐studyregister.com/study/4036 (accessed 12 December 2018).
-
- NCT01171963. Efficacy, immunogenicity and safety of two doses of GlaxoSmithKline (GSK) Biologicals' oral live attenuated liquid human rotavirus (HRV) vaccine (444563), in healthy infants. clinicaltrials.gov/show/NCT01171963 (first received 29 July 2010).
RV1 Madhi 2010‐AF {published data only}
-
- Madhi SA, Cunliffe NA, Steele D, Witte D, Kirsten M, Louw C, et al. Effect of human rotavirus vaccine on severe diarrhea in African infants. New England Journal of Medicine 2010;362(4):289‐98. - PubMed
-
- Madhi SA, Kirsten M, Louw C, Bos P, Aspinall S, Bouckenooghe A, et al. Efficacy and immunogenicity of two or three dose rotavirus‐vaccine regimen in South African children over two consecutive rotavirus seasons: a randomized, double‐blind, placebo‐controlled trial. Vaccine 2012;30(Suppl 1):A44‐51. - PubMed
-
- NCT00241644. Multi‐center study to assess the efficacy, safety and immunogenicity of 2 or 3 doses of GSK Biologicals' oral live attenuated human rotavirus (HRV) vaccine given concomitantly with routine EPI vaccinations in healthy infants. clinicaltrials.gov/show/NCT00241644 (first received 19 October 2005).
-
- NCT00598468. Multi‐center study to assess efficacy, safety & immunogenicity of 2 or 3 doses of GSK Biologicals' oral live attenuated human rotavirus (HRV) vaccine given concomitantly with routine EPI vaccinations in healthy infants. clinicaltrials.gov/show/NCT00598468 (first received 19 October 2005).
RV1 Madhi 2010‐MWI {published data only}
-
- Madhi SA, Cunliffe NA, Steele D, Witte D, Kirsten M, Louw C, et al. Effect of human rotavirus vaccine on severe diarrhea in African infants. New England Journal of Medicine 2010;362(4):289‐98. - PubMed
-
- NCT00241644. Multi‐center study to assess the efficacy, safety and immunogenicity of 2 or 3 doses of GSK Biologicals' oral live attenuated human rotavirus (HRV) vaccine given concomitantly with routine EPI vaccinations in healthy infants. clinicaltrials.gov/show/NCT00241644 (first received 19 October 2005).
-
- NCT00598468. Multi‐center study to assess efficacy, safety & immunogenicity of 2 or 3 doses of GSK Biologicals' oral live attenuated human rotavirus (HRV) vaccine given concomitantly with routine EPI vaccinations in healthy infants. clinicaltrials.gov/show/NCT00598468 (first received 19 October 2005).
RV1 Madhi 2010‐ZAF {published data only}
-
- Madhi SA, Cunliffe NA, Steele D, Witte D, Kirsten M, Louw C, et al. Effect of human rotavirus vaccine on severe diarrhea in African infants. New England Journal of Medicine 2010;362(4):289‐98. - PubMed
-
- Madhi SA, Kirsten M, Louw C, Bos P, Aspinall S, Bouckenooghe A, et al. Efficacy and immunogenicity of two or three dose rotavirus‐vaccine regimen in South African children over two consecutive rotavirus seasons: a randomized, double‐blind, placebo‐controlled trial. Vaccine 2012;30(Suppl 1):A44‐51. - PubMed
-
- NCT00241644. Multi‐center study to assess the efficacy, safety and immunogenicity of 2 or 3 doses of GSK Biologicals' oral live attenuated human rotavirus (HRV) vaccine given concomitantly with routine EPI vaccinations in healthy infants. clinicaltrials.gov/show/NCT00241644 (first received 19 October 2005).
-
- NCT00598468. Multi‐center study to assess efficacy, safety & immunogenicity of 2 or 3 doses of GSK Biologicals' oral live attenuated human rotavirus (HRV) vaccine given concomitantly with routine EPI vaccinations in healthy infants. clinicaltrials.gov/show/NCT00598468 (first received 19 October 2005).
RV1 Narang 2009‐IND {published data only}
-
- GlaxoSmithKline[103792‐044]. A phase IIIb, randomised, multicentre double‐blind, placebo‐controlled study of the immunogenicity and safety of two doses of GlaxoSmithKline (GSK) Biologicals’ oral live attenuated human rotavirus (HRV) vaccine (RIX4414) as primary dosing in healthy infants in India of approximately 8 weeks of age at the first dose. www.gsk‐studyregister.com/study/2723 (accessed 12 December 2018).
-
- NCT00289172. A multicenter study of the immunogenicity & safety of 2 doses of GSK Biologicals’ oral live attenuated human rotavirus vaccine (RIX4414) as primary dosing of healthy infants in India aged approximately 8 wks at the time of the first dose. clinicaltrials.gov/ct2/show/record/NCT00289172 (first received 9 February 2006).
-
- Narang A, Bose A, Pandit AN, Dutta P, Kang G, Bhattacharya SK, et al. Immunogenicity, reactogenicity and safety of human rotavirus vaccine (RIX4414) in Indian infants. Human Vaccines 2009;5(6):414‐9. - PubMed
RV1 NCT00158756‐RUS {published data only}
-
- GlaxoSmithKline[104021‐DTPw CSL‐HBVGOD‐005]. A phase III, partially blind, randomized study to evaluate the immunogenicity, safety and reactogenicity of GlaxoSmithKline (GSK) Biologicals’ Tritanrix™‐HepB and GSK Biologicals Kft’s DTPw‐HBV vaccines as compared to concomitant administration of Commonwealth Serum Laboratory’s (CSL’s) DTPw (Triple Antigen™) and GSK Biologicals’ HBV (Engerix™‐B), when co‐administered with GSK Biologicals’ oral live attenuated human rotavirus (HRV) vaccine, to healthy infants at 3, 4½ and 6 months of age, after a birth dose of hepatitis B vaccine. www.gsk‐studyregister.com/study/2753 (accessed 12 December 2018).
-
- NCT00158756. Assess immunogenicity & reactogenicity of 2 formulations of GSK's DTPw‐HBV vaccines vs concomitant admn of CSL's DTPw & GSK's HBV vaccine, co‐admnd with GSK's rotavirus vaccine, to infants at 3, 4½ & 6 mths, after birth dose of HBV vaccine. clinicaltrials.gov/show/NCT00158756 (first received 12 September 2005).
RV1 Omenaca 2012‐EU {published data only}
-
- GlaxoSmithKline[106481‐054]. Phase IIIb, Double Blind, Randomised, Placebo‐Controlled, Multi‐Country/Centre, Study to Assess Safety, Reactogenicity & Immunogenicity of 2 Doses of GSK Biologicals' Oral Live Attenuated Human Rotavirus (HRV) Vaccine in Pre‐Term Infants. www.gsk‐studyregister.com/study/3064 (accessed 12 December 2018).
-
- NCT00420745. Phase IIIb, double blind, randomised, placebo‐controlled, multi‐country/centre, study to assess safety, reactogenicity & immunogenicity of 2 doses of GSK Biologicals' oral live attenuated human rotavirus (HRV) vaccine in pre‐term infants. clinicaltrials.gov/show/NCT00420745 (first received 11 January 2007).
-
- Omenaca F, Sarlangue J, Szenborn L, Nogueira M, Suryakiran PV, Smolenov IV, et al. Safety, reactogenicity and immunogenicity of the human rotavirus vaccine in preterm European infants: a randomized phase IIIb study. Pediatric Infectious Disease Journal 2012;31(5):487‐93. - PubMed
RV1 Phua 2005‐SGP {published data only}
-
- Vos B, Gillard P, Cheuvart B. RIX4414 vaccine efficacy against rotavirus gastroenteritis due to G2P[4] strain. 46th Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC); 2006 September 27‐30; San Francisco, USA. 2006. [Not available for review]
-
- Vos B, Vesikari T, Linhares AC, Salinas B, Perez‐Schael I, Ruiz‐Palacios GM, et al. A rotavirus vaccine for prophylaxis of infants against rotavirus gastroenteritis. Pediatric Infectious Disease Journal 2004;23(10 Suppl):179‐82. [No data for review] - PubMed
-
- Emmanuel B, et al. Immunogenicity of an acellular pertussis combination vaccine co‐administered with a novel rotavirus vaccine in Singaporean infants. Asian Congress on Pediatric Infectious Diseases (ACPID); 2‐4 September 2004; Kota Kinabula, Malaysia 2004. [Not available for review]
-
- Emmanuel S, Phua KB, Goh P, Quak SH, Datta SK, Han HH, et al. Immunogenicity of an acellular pertussis combination vaccine co‐administered with a novel rotavirus vaccine in Singaporean infants. 23rd Annual Meeting of European Society for Paediatric Infectious Diseases (ESPID); 2005 May 18‐20; Valencia, Spain. 2005. [Not available for review]
-
- GlaxoSmithKline[444563‐007]. A phase IIb, double‐blind, randomized, placebo‐controlled study to assess the efficacy, immunogenicity, reactogenicity and safety of two doses of GlaxoSmithKline (GSK) Biologicals’ oral live attenuated human rotavirus (HRV) vaccine at different viral concentrations (104.7, 105.2 and 106.1 foci forming units (ffu)) in healthy infants previously uninfected with human rotavirus and approximately three months of age, when administered concurrently with DTPa‐IPV/Hib and HBV vaccines. www.gsk‐studyregister.com/study/6785 (accessed 12 December 2018).
RV1 Phua 2009‐AS {published data only}
-
- Lau YL, Nelson EA, Poon KH, Chan PK, Chiu S, Sung R, et al. Efficacy, safety and immunogenicity of a human rotavirus vaccine (RIX4414) in Hong Kong children up to three years of age: a randomized, controlled trial. Vaccine 2013;31(18):2253‐9. - PubMed
-
- NCT00197210. A phase III, double‐blind, randomized, placebo‐controlled, multi‐country and multi‐center study to assess the efficacy and safety of two doses of GSK Biologicals' oral live attenuated human rotavirus (HRV) vaccine in healthy infants. clinicaltrials.gov/show/NCT00197210 (first received 20 September 2005).
-
- NCT00329745. A phase III, double‐blind, randomized, placebo‐controlled, multi‐country and multi‐center study to assess the efficacy and safety of two doses of GSK Biologicals' oral live attenuated human rotavirus (HRV) vaccine in healthy infants. clinicaltrials.gov/show/NCT00329745 (first received 25 May 2006).
-
- Phua KB, Lim FS, Lau YL, Nelson EA, Huang LM, Quak SH, et al. Rotavirus vaccine RIX4414 efficacy sustained during the third year of life: A randomized clinical trial in an Asian population. Vaccine 2012;30(30):4552‐7. - PubMed
-
- Phua KB, Lim FS, Lau YL, Nelson EA, Huang LM, Quak SH, et al. Safety and efficacy of human rotavirus vaccine during the first 2 years of life in Asian infants: randomised, double‐blind, controlled study. Vaccine 2009;27(43):5936‐41. - PubMed
RV1 Rivera 2011‐DOM {published data only}
-
- EUCTR2015‐001542‐29. Immunization of infants 6‐14 weeks of age, with GlaxoSmithKline Biologicals Rotavirus vaccine to explore the existence of transmission of rotavirus vaccine strain between twins in a family. clinicaltrialsregister.eu/ctr‐search/trial/2015‐001542‐29/results (accessed 31 August 2017).
-
- NCT00396630. A Phase IIIb, randomized, double‐blind, placebo‐controlled study to explore the existence of horizontal transmission of the RIX4414 vaccine strain between twins within a family. clinicaltrials.gov/show/NCT00396630 (first received 7 November 2006).
-
- Rivera L, Peña LM, Stainier I, Gillard P, Cheuvart B, Smolenov I, et al. Horizontal transmission of a human rotavirus vaccine strain‐‐a randomized, placebo‐controlled study in twins. Vaccine 2011;29(51):9508‐13. - PubMed
RV1 Ruiz‐Palac 06‐LA/EU {published data only}
-
- Costa Clemens SA, et al. Operational organization of a large scale phase III clinical trial of rotavirus vaccine in multiple sites and countries in Latin America. International Congress of Pediatrics (ICP); 2004 August 15‐20; Cancun, Mexico. 2004. [Not available for review]
-
- Vos B, et al. Rotarix™: an effective way to prevent rotavirus diarrhoea and vomiting. 9th Congress of the Asian Pan Pacific Society of Paediatric Gastroenterology, Hepatology and Nutrition & 27th Annual Congress of the Malaysian Paediatric Association; 2005 June 16‐19; Kuala Lumpur, Malaysia. 2005. [Not available for review]
-
- GlaxoSmithKline[444563‐023‐pt1]. A phase III, double‐blind, randomized, placebo‐controlled, multi‐country and multi‐center study to assess the efficacy, safety and immunogenicity of two doses of GSK Biologicals’ oral live attenuated human rotavirus (HRV) vaccine in healthy infants. (Efficacy data from Visit 1 to Visit 4). www.gsk‐studyregister.com/study/6791 (accessed 12 December 2018).
-
- GlaxoSmithKline[444563‐023‐pt2]. A phase III, double‐blind, randomized, placebo‐controlled, multi‐country and multi‐center study to assess the efficacy, safety and immunogenicity of two doses of GSK Biologicals’ oral live attenuated human rotavirus (HRV) vaccine in healthy infants. (2nd year efficacy from Visit 4 to Visit 6). www.gsk‐studyregister.com/study/6791 (accessed 12 December 2018).
RV1 Salinas 2005‐LA {published data only}
-
- Araujo EC, Clemens SA, Oliveira CS, Justino MC, Rubio P, Gabbay YB, et al. Safety, immunogenicity, and protective efficacy of two doses of RIX4414 live attenuated human rotavirus vaccine in healthy infants. Jornal de Pediatria 2007;83(3):217‐24. - PubMed
-
- Vos B, Hardt K, Linhares AC, Ruiz‐Palacios G, Guerrero L, Salinas B. Efficacy of two doses of a human monovalent rotavirus vaccine, Rotarix™ in preventing gastro‐enteritis due to G1 and Non‐G1 rotavirus in Brazil, Mexico and Venezuela [Presentation]. 8th International Symposium on Double‐Stranded RNA Viruses; 2003 September 13‐18; Lucca, Italy. 2003. [Not available for review]
-
- Vos B, Vesikari T, Linhares AC, Salinas B, Perez‐Schael I, Ruiz‐Palacios GM, et al. A rotavirus vaccine for prophylaxis of infants against rotavirus gastroenteritis. Pediatric Infectious Disease Journal 2004;23(10 Suppl):179‐82. - PubMed
-
- GlaxoSmithKline[444563‐006‐Annex]. A phase IIb, double‐blind, randomized, placebo‐controlled study to assess the efficacy, immunogenicity, reactogenicity and safety of two doses of GlaxoSmithKline (GSK) Biologicals' live attenuated human rotavirus (HRV) vaccine at different virus concentrations (104.7, 105.2 and 105.8 foci forming units [ffu]) in healthy infants (approximately 2 months of age at first dose) following a 0, 2 month schedule and previously uninfected with HRV, when administered concurrently with DTPw‐HBV and Hib vaccines. [This summary presents results for the second and combined efficacy periods and results from the 3‐Dose subset. Results from the first efficacy period are presented in 444563/006 (Rota‐006) summary.]. www.gsk‐studyregister.com/study/6784 (accessed 12 December 2018).
-
- GlaxoSmithKline[444563‐006]. A phase IIb, double‐blind, randomized, placebo‐controlled study to assess the efficacy, immunogenicity, reactogenicity and safety of two doses of GlaxoSmithKline (GSK) Biologicals' live attenuated human rotavirus (HRV) vaccine at different virus concentrations (104.7, 105.2 and 105.8 foci forming units [ffu]) in healthy infants (approximately 2 months of age at first dose) following a 0, 2 month schedule and previously uninfected with HRV, when administered concurrently with DTPw‐HBV and Hib vaccines. www.gsk‐studyregister.com/study/6784 (accessed 12 December 2018).
RV1 Steele 2008‐ZAF {published data only}
-
- GlaxoSmithKline[444563‐014]. A phase II, double‐blind before the 2002 rotavirus season and single blind with respect to OPV after, randomised, placebo‐controlled study of the safety, reactogenicity and immunogenicity of two doses of GSK Biologicals’ oral live attenuated human rotavirus (HRV) vaccine (RIX4414 at 105 ffu) co‐administered with either oral polio vaccine (OPV) or inactivated polio vaccine (IPV) in healthy infants (approximately 5‐10 weeks old) in South Africa. www.gsk‐studyregister.com/study/6787 (accessed 12 December 2018).
-
- ISRCTN37373664. A double blind, randomised placebo controlled study of the safety, reactogenicity and immunogenicity of two doses of orally administered human rotavirus vaccine (RIX4414) in healthy infants in South Africa. www.controlled‐trials.com/ISRCTN37373664 (first received 25 November 2005).
-
- NCT00346892. Reactogenicity & immunogenicity study of two doses of GSK Biologicals’ oral live attenuated HRV vaccine co‐administered with either OPV or IPV in healthy infants (approximately 5‐10 weeks old) in South Africa. clinicaltrials.gov/show/NCT00346892 (first received 30 June 2006).
-
- Steele AD, Tumbo J, Armah G, Reynders J, Scholtz F, Bos P, et al. Immunogenicity and reactogenicity of a new live attenuated oral rotavirus vaccine (RIX4414) when administered concurrently with poliovirus vaccines in African infants. 24th International Congress of Pediatrics; 2004 August 15‐20; Cancun, Mexico. 2004. [Not available for review]
-
- Steele AD, Tumbo JM, Armah GE, Reynders J, Scholtz F, Bos P, et al. Concomitant administration of a live‐attenuated oral rotavirus vaccine (RIX4414) with poliovirus vaccines in African infants. 23rd Annual Meeting of the European Society for Pediatric Infectious Diseases‐ESPID; 2005 May 18‐20; Valencia, Spain 2005. [Not available for review]
RV1 Steele 2010a‐ZAF {published data only}
-
- EUCTR2015‐001484‐39. A phase II study to assess the safety and immunogenicity of GlaxoSmithKline Biologicals rotavirus vaccine, RIX4414 when administered to HIV infected infants in South Africa. www.clinicaltrialsregister.eu/ctr‐search/trial/2015‐001484‐39/results (first received 11 June 2015).
-
- GlaxoSmithKline[444563‐022]. A phase II, double‐blind, randomized, placebo‐controlled study to assess the safety, reactogenicity and immunogenicity of three doses of GlaxoSmithKline (GSK) Biologicals’ oral live attenuated human rotavirus (HRV) vaccine. www.gsk‐studyregister.com/study/6790 (accessed 12 December 2018).
-
- ISRCTN11877362. A phase II, double‐blind, randomised, placebo‐controlled study to assess the safety, reactogenicity and immunogenicity of three doses of GlaxoSmithKline (GSK) Biologicals’ oral live attenuated human rotavirus (HRV) vaccine (RIX4414 at 106.5 CCID50) administered to human immunodeficiency virus (HIV) infected infants at 6, 10 and 14 weeks of age in South Africa. www.controlled‐trials.com/ISRCTN11877362 (first received 25 November 2005).
-
- NCT00263666. A study of safety, reactogenicity and immunogenicity of HRV vaccine in HIV infected infants in South Africa. clinicaltrials.gov/ct2/show/results/NCT00263666 (first received on 9 December 2005).
-
- Steele AD, Madhi SA, Louw CE, Bos P, Tumbo JM, Werner CM, et al. Safety, reactogenicity, and immunogenicity of human rotavirus vaccine RIX4414 in human immunodeficiency virus‐positive infants in South Africa. Pediatric Infectious Diseases Journal 2011;30(2):125‐30. - PubMed
RV1 Steele 2010b‐ZAF {published data only}
-
- GlaxoSmithKline[444563‐013]. A phase II, double‐blind before the 2002 rotavirus season and single blind with respect to OPV after, randomised, placebo‐controlled study of the safety, reactogenicity and immunogenicity of two doses of GSK Biologicals’ oral live attenuated human rotavirus (HRV) vaccine (RIX4414 at 105 ffu) co‐administered with either oral polio vaccine (OPV) or inactivated polio vaccine (IPV) in healthy infants (approximately 5‐10 weeks old) in South Africa. www.gsk‐studyregister.com/study/6786 (accessed 12 December 2018).
-
- ISRCTN37373664. A double blind, randomised placebo controlled study of the safety, reactogenicity and immunogenicity of two doses of orally administered human rotavirus vaccine (RIX4414) in healthy infants in South Africa. controlled‐trials.com/ISRCTN37373664 (first received 25 November 2005).
-
- NCT00383903. A study of the safety, reactogenicity and immunogenicity of 2 or 3 doses of GSK Biologicals’ oral live attenuated human rotavirus (HRV) vaccine in healthy infants (approximately 5‐10 weeks old) in South Africa. clinicaltrials.gov/show/NCT00383903 (accessed 4 October 2006).
-
- Steele AD, Reynders J, Scholtz F, Bos P, De Beer MC, Tumbo J, et al. Comparison of 2 different regimens for reactogenicity, safety, and immunogenicity of the live attenuated oral rotavirus vaccine RIX4414 coadministered with oral polio vaccine in South African infants. Journal of Infectious Diseases 2010;202(Suppl):S93‐100. - PubMed
RV1 Tregnaghi 2011‐LA {published data only}
-
- GlaxoSmithKline[444563‐024]. A phase III, double‐blind, randomized, placebo‐controlled, multi‐country and multi‐center study to assess the efficacy, immunogenicity and safety of two doses of GSK Biologicals’ oral live attenuated human rotavirus (HRV) vaccine given concomitantly with routine EPI vaccinations including OPV in healthy infants. www.gsk‐studyregister.com/study/6792 (accessed 12 December 2018).
-
- Gonzalez Ayala S, Rivera L, Rivera‐Medina DM, Lopez P, Valencia A, León T, et al. Co‐administration with rotavirus vaccine rix4414 (Rotarix™) does not interfere with the immunogenicity of oral polio vaccine (OPV). [Poster]. World Society for Pediatric Infectious Diseases (WSPID); 2007 November 15‐18; Bangkok, Thailand. 2007. [Not available for review]
-
- Tregnaghi MW, Abate HJ, Valencia A, Lopez P, Silveira TR, Rivera L, et al. Human rotavirus vaccine is highly efficacious when coadministered with routine expandedprogram of immunization vaccines including oral poliovirus vaccine in Latin America. Pediatric Infectious Disease Journal 2011;30(6):e103‐e8. - PubMed
RV1 Vesikari 2004a‐FIN {published data only}
-
- GlaxoSmithKline[444563‐003]. A phase II, double‐blind, randomized, placebo‐controlled, dose‐escalating, stepwise study to assess safety, reactogenicity and immunogenicity of GlaxoSmithKline Biologicals’ live attenuated human rotavirus (HRV) vaccine in healthy infants previously uninfected with human rotavirus. www.gsk‐studyregister.com/study/6781 (accessed 12 December 2018).
-
- Vesikari T, Karvonen A, Korhonen T, Espo M, Lebacq E, Forster J, et al. Safety and immunogenicity of RIX4414 live attenuated human rotavirus vaccine in adults, toddlers and previously uninfected infants. Vaccine 2004;22(21‐2):2836‐42. - PubMed
RV1 Vesikari 2004b‐FIN {published data only}
-
- Vos B, Vesikari T, Linhares AC, Salinas B, Perez‐Schael I, Ruiz‐Palacios GM, et al. A rotavirus vaccine for prophylaxis of infants against rotavirus gastroenteritis. Pediatric Infectious Disease Journal 2004;23(10 Suppl):179‐82. - PubMed
-
- GlaxoSmithKline[444563‐004‐Annex]. A phase IIb, double‐blind, randomized, placebo‐controlled study to assess the efficacy, immunogenicity, reactogenicity and safety of two doses of GSK Biologicals’ oral live attenuated human rotavirus (HRV) vaccine in healthy infants approximately 2 months of age and previously uninfected with HRV. [This summary presents results for the second efficacy period (from the end of the first rotavirus season post‐vaccination until the end of the second rotavirus season) and for the two consecutive rotavirus seasons.]. www.gsk‐studyregister.com/study/6782 (accessed 12 December 2018).
-
- GlaxoSmithKline[444563‐004]. A phase IIb, double‐blind, randomized, placebo‐controlled study to assess the efficacy, immunogenicity, reactogenicity and safety of two doses of GSK Biologicals’ oral live attenuated human rotavirus (HRV) vaccine in healthy infants approximately 2 months of age and previously uninfected with HRV. [Results for the first efficacy period (starting from 2 weeks after Dose 2 until the end of the first RV season following vaccination)]. www.gsk‐studyregister.com/study/6782 (accessed 12 December 2018).
-
- NCT00425737. A study to assess the efficacy, immunogenicity and safety of two doses of oral live attenuated human rotavirus (HRV) vaccine (Rotarix) in healthy infants. clinicaltrials.gov/show/NCT00425737 (first receiveded 23 January 2007).
-
- Vesikari T, Karvonen A, Espo M, Korhonen T, Delem A, Vos B. Efficacy evaluation of an oral human rotavirus (HRV) vaccine in previously uninfected Finnish infants. 42nd Interscience Conference on Antimicrobial Agents and Chemotherapy; 2002 September 27‐30; San Diego, California. 2002. [Not available for review]
RV1 Vesikari 2007a‐EU {published data only}
-
- GlaxoSmithKline[102247‐036‐Annex]. A phase IIIb, double‐blind, randomized, placebo‐controlled, multi‐country and multi‐center study to assess the efficacy, safety and immunogenicity of two doses of GlaxoSmithKline (GSK) Biologicals’ oral live attenuated human rotavirus (HRV) vaccine in healthy infants in co‐administration with specific childhood vaccinations. [This summary presents results for the second and combined efficacy periods]. www.gsk‐studyregister.com/study/2669 (accessed 12 December 2018).
-
- GlaxoSmithKline[102247‐036‐Yr3]. A phase IIIb, double‐blind, randomized, placebo‐controlled, multi‐country and multi‐center study to assess the efficacy, safety and immunogenicity of two doses of GlaxoSmithKline (GSK) Biologicals’ oral live attenuated human rotavirus (HRV) vaccine in healthy infants in co‐administration with specific childhood vaccinations. [A phase IIIb open study to assess the long‐term efficacy and safety of GlaxoSmithKline (GSK) Biologicals’ oral live attenuated human rotavirus (HRV) vaccine approximately three years after vaccination in healthy infants aged 6‐12 weeks at the time of first vaccination in the Rota‐036 study (eTrack No.102247) in Finland.]. www.gsk‐studyregister.com/study/2669 (accessed 12 December 2018).
-
- GlaxoSmithKline[102247‐036]. A phase IIIb, double‐blind, randomized, placebo‐controlled, multi‐country and multi‐center study to assess the efficacy, safety and immunogenicity of two doses of GlaxoSmithKline (GSK) Biologicals’ oral live attenuated human rotavirus (HRV) vaccine in healthy infants in co‐administration with specific childhood vaccinations. [This summary presents results from the first efficacy period.] [102247‐036]. www.gsk‐studyregister.com/study/2669 (accessed 12 December 2018).
-
- NCT00140686. A multi‐country & multi‐center study to assess the efficacy, safety & immunogenicity of 2 doses of GSK Biologicals’ oral live attenuated human rotavirus (HRV) vaccine in healthy infants in co‐administration with specific childhood vaccines. clinicaltrials.gov/ct2/show/record/NCT00140686 (first received 1 September 2005). [Trial registration document]
-
- NCT00420316. To assess long‐term efficacy & safety of subjects approximately 3 years after priming with 2 doses of GlaxoSmithKline (GSK) Biologicals' oral live attenuated human rotavirus (HRV) vaccine (Rotarix) in the primary vaccination study (102247). clinicaltrials.gov/show/NCT00420316 (first received 11 January 2007).
RV1 Vesikari 2011‐FIN {published data only}
-
- GlaxoSmithKline[104480‐048]. A phase II, double‐blind, randomized, placebo controlled study to compare the immunogenicity, reactogenicity and safety of two different formulations of GlaxoSmithKline (GSK) Biologicals’ live attenuated human rotavirus (HRV) vaccine given as a two‐dose primary vaccination in healthy infants previously uninfected with HRV. www.gsk‐studyregister.com/study/2783 (accessed 12 December 2018).
-
- NCT00137930. Compare the immunogenicity, reactogenicity & safety of 2 different formulations of GSK Biologicals’ live attenuated human rotavirus (HRV) vaccine given as a two‐dose primary vaccination in healthy infants previously uninfected with HRV. clinicaltrials.gov/ct2/show/record/NCT00137930 (first received 30 August 2005).
-
- Vesikari T, Karvonen A, Bouckenooghe A, Suryakiran PV, Smolenov I, Han HH. Immunogenicity, reactogenicity and safety of the human rotavirus vaccine RIX4414 oral suspension (liquid formulation) in Finnish infants. Vaccine 2011;29(11):2079‐84. - PubMed
-
- Vesikari T, et al. Immunogenicity of liquid formulation of the oral live attenuated human rotavirus vaccine (Rotarix™) [Poster]. Sociedad Latinoamericana de Infectología Pediátrica (SLIPE). San Jose, Costa Rica 8‐11 May 2007. [Not available for review]
RV1 Ward 2006‐USA {published data only}
-
- Ward RL, Kirkwood CD, Sander DS, Smith VE, Shao M, Bean JA, et al. Reductions in cross‐neutralizing antibody responses in infants after attenuation of the human rotavirus vaccine candidate 89‐12. Journal of Infectious Diseases 2006;194(12):1729‐36. - PubMed
RV1 Zaman 2009‐BGD {published data only}
-
- GlaxoSmithKline[103992‐045]. A phase II, randomised, double‐blind, placebo‐controlled study to evaluate the immunogenicity, reactogenicity and safety of two doses of GSK Biologicals’ oral live attenuated human rotavirus (HRV) vaccine (RIX4414 at 106.5 CCID50) when given concomitantly with OPV versus when given alone (HRV vaccine dose given 15 days after the OPV dose) in healthy infants in Bangladesh. www.gsk‐studyregister.com/study/2748 (accessed 12 December 2018).
-
- NCT00139334. Evaluate immunogenicity, reactogenicity & safety of 2 doses of GSK Biologicals’ oral live attenuated HRV vaccine (RIX4414 at 106.5 CCID50) when given concomitantly with OPV versus given alone (HRV vaccine dose given 15 days after the OPV dose) in healthy infants in Bangladesh. clinicaltrials.gov/show/NCT00139334 (first received 31 August 2005).
-
- Zaman K, Sack DA, Yunus M, Arifeen SE, Podder G, Azim T, et al. Bangladesh Rotavirus Vaccine study group. Successful co‐administration of a human rotavirus and oral poliovirus vaccines in Bangladeshi infants in a 2‐dose schedule at 12 and 16 weeks of age. Vaccine 2009;27(9):1333‐9. - PubMed
RV1 Zaman 2017‐BGD {published data only}
RV5 Armah 2010‐AF {published data only}
-
- Armah GE, Breiman RF, Tapia MD, Dallas MJ, Neuzil KM, Binka FN, et al. Immunogenicity of the pentavalent rotavirus vaccine in African infants. Vaccine 2012;30(Suppl 1):A86‐93. - PubMed
-
- Armah GE, Sow SO, Breiman RF, Dallas MJ, Tapia MD, Feikin DR, et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in sub‐Saharan Africa: a randomised, double‐blind, placebo‐controlled trial. Lancet 2010;376(9741):606‐14. - PubMed
-
- Breiman RF, Zaman K, Armah G, Sow SO, Anh DD, Victor JC, et al. Analyses of health outcomes from the 5 sites participating in the Africa and Asia clinical efficacy trials of the oral pentavalent rotavirus vaccine. Vaccine 2012;30(Suppl 1):A24‐9. - PubMed
-
- Gruber JF, Hille DA, Liu GF, Kaplan SS, Nelson M, Goveia MG, et al. Heterogeneity of rotavirus vaccine efficacy among infants in developing countries. Pediatric Infectious Disease Journal 2017;36(1):72‐8. - PubMed
RV5 Armah 2010‐GHA {published data only}
-
- Armah GE, Sow SO, Breiman RF, Dallas MJ, Tapia MD, Feikin DR, et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in sub‐Saharan Africa: a randomised, double‐blind, placebo‐controlled trial. Lancet 2010;376(9741):606‐14. - PubMed
-
- Tapia MD, Armah G, Breiman RF, Dallas MJ, Lewis KD, Sow SO, et al. Secondary efficacy endpoints of the pentavalent rotavirus vaccine against gastroenteritis in sub‐Saharan Africa. Vaccine 2012;30(Suppl 1):A79‐85. - PubMed
RV5 Armah 2010‐KEN {published data only}
-
- Armah GE, Sow SO, Breiman RF, Dallas MJ, Tapia MD, Feikin DR, et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in sub‐Saharan Africa: a randomised, double‐blind, placebo‐controlled trial. Lancet 2010;376(9741):606‐14. - PubMed
-
- Feikin DR, Laserson KF, Ojwando J, Nyambane G, Ssempijja V, Audi A, et al. Efficacy of pentavalent rotavirus vaccine in a high HIV prevalence population in Kenya. Vaccine 2012;30(Suppl 1):A52‐60. - PubMed
-
- Laserson KF, Nyakundi D, Feikin DR, Nyambane G, Cook E, Oyieko J, et al. Safety of the pentavalent rotavirus vaccine (PRV), RotaTeq((R)), in Kenya, including among HIV‐infected and HIV‐exposed infants. Vaccine 2012;30(Suppl 1):A61‐70. - PubMed
-
- Tapia MD, Armah G, Breiman RF, Dallas MJ, Lewis KD, Sow SO, et al. Secondary efficacy endpoints of the pentavalent rotavirus vaccine against gastroenteritis in sub‐Saharan Africa. Vaccine 2012;30(Suppl 1):A79‐85. - PubMed
RV5 Armah 2010‐MLI {published data only}
-
- Armah GE, Sow SO, Breiman RF, Dallas MJ, Tapia MD, Feikin DR, et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in sub‐Saharan Africa: a randomised, double‐blind, placebo‐controlled trial. Lancet 2010;376(9741):606‐14. - PubMed
-
- Sow SO, Tapia M, Haidara FC, Ciarlet M, Diallo F, Kodio M, et al. Efficacy of the oral pentavalent rotavirus vaccine in Mali. Vaccine 2012;30(Suppl 1):A71‐8. - PubMed
-
- Tapia MD, Armah G, Breiman RF, Dallas MJ, Lewis KD, Sow SO, et al. Secondary efficacy endpoints of the pentavalent rotavirus vaccine against gastroenteritis in sub‐Saharan Africa. Vaccine 2012;30(Suppl 1):A79‐85. - PubMed
RV5 Block 2007‐EU/USA {published data only}
-
- Block SL, Vesikari T, Goveia MG, Rivers SB, Adeyi BA, Dallas MJ, et al. Efficacy, immunogenicity, and safety of a pentavalent human‐bovine (WC3) reassortant rotavirus vaccine at the end of shelf life. Pediatrics 2007;119(1):11‐8. - PubMed
-
- Merck[PN‐007]. Study of the efficacy, safety, and immunogenicity of RotaTeq™ at expiry potency NCT00092443 (PN 007). www.clinicalstudyresults.org/drugdetails/?indication_id=523&sort=c.c... 2008.
-
- NCT00092443. Study of the efficacy, safety, and immunogenicity of V260 at expiry. clinicaltrials.gov/ct2/show/NCT00092443 (first received 27 September 2004). [Trial registration document]
RV5 Ciarlet 2009‐EU {published and unpublished data}
-
- Ciarlet M, He S, Lai S, Petrecz M, Yuan G, Liu GF, et al. Concomitant use of the 3‐dose oral pentavalent rotavirus vaccine with a 3‐dose primary vaccination course of a diphtheria‐tetanus‐acellular pertussis‐hepatitis B‐inactivated polio‐Haemophilus influenzae type b vaccine: immunogenicity and reactogenicity. Pediatric Infectious Disease Journal 2009;28(3):177‐81. - PubMed
-
- Merck[PN‐010]. Safety and immunogenicity of concomitant use of RotaTeq™ and INFANRIX™ hexa in healthy infants NCT00258154 (PN 010) [Merck & Co., Inc. Study Synopsis]. www.clinicalstudyresults.org/drugdetails/?indication_id=1101&sort=c.... 2008.
-
- NCT00258154. Safety and immunogenicity of concomitant use of V260 and INFANRIX(Tm) hexa in healthy infants. clinicaltrials.gov/show/NCT00258154 (first received 24 November 2005). [Trial registration document (no data for review)]
RV5 Clark 2003‐USA {published and unpublished data}
-
- Clark HF, Burke CJ, Volkin DB, Offit P, Ward RL, Bresee JS, et al. Safety, immunogenicity and efficacy in healthy infants of G1 and G2 human reassortant rotavirus vaccine in a new stabilizer/buffer liquid formulation. Pediatric Infectious Disease Journal 2003;22(10):914‐20. - PubMed
RV5 Clark 2004‐USA {published and unpublished data}
-
- Clark H, White C, Offit P, Stinson D, Eiden J, Weaver S, et al. Preliminary evaluation of safety and efficacy of quadrivalent human‐bovine reassortant rotavirus vaccine [abstract]. Pediatric Research 1995;37:172A. [No data for review]
-
- Clark HF, Bernstein DI, Dennehy PH, Offit P, Pichichero M, Treanor J, et al. Safety, efficacy, and immunogenicity of a live, quadrivalent human‐bovine reassortant rotavirus vaccine in healthy infants. Journal of Pediatrics 2004;144(2):184‐90. - PubMed
-
- Clark HF, Offit PA, Ellis RW, Eiden JJ, Krah D, Shaw AR, et al. The development of multivalent bovine rotavirus (strain WC3) reassortant vaccine for infants. Journal of Infectious Diseases 1996;174(Suppl 1):73‐80. [No data for review] - PubMed
-
- Ward RL, Bernstein DI, Smith VE, Sander DS, Shaw A, Eiden JJ, et al. Rotavirus immunoglobulin a responses stimulated by each of 3 doses of a quadrivalent human/bovine reassortant rotavirus vaccine. Journal of Infectious Diseases 2004;189(12):2290‐3. - PubMed
RV5 Dhingra 2014‐IND {published data only}
-
- CTRI/2012/07/002820. A study to evaluate safety of Rotavirus vaccine in healthy adult volunteers followed by safety, tolerability and immunogenicity evaluation in healthy infants. ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=4929&EncHid=&us... 2012 (accessed 11 June 2017).
-
- Dhingra MS, Kundu R, Gupta M, Kanungo S, Ganguly N, Singh MP, et al. Evaluation of safety and immunogenicity of a live attenuated tetravalent (G1‐G4) Bovine‐Human Reassortant Rotavirus vaccine (BRV‐TV) in healthy Indian adults and infants. Vaccine 2014;32 Suppl 1:A117‐23. - PubMed
RV5 Iwata 2013‐JPN {published and unpublished data}
-
- NCT00718237. A Phase III randomized, placebo‐controlled clinical trial to study the efficacy and safety of V260 in healthy infants in Japan. clinicaltrials.gov/ct2/show/record/NCT00718237 (accessed 18 July 2008).
RV5 Kim 2008‐KOR {published and unpublished data}
-
- Kim DS, Lee TJ, Kang JH, Kim JH, Lee JH, Ma SH, et al. Immunogenicity and safety of a pentavalent human‐bovine (WC3) reassortant rotavirus vaccine in healthy infants in Korea. Pediatric Infectious Disease Journal 2008;27(2):177‐8. - PubMed
-
- Merck[PN‐013]. Immunogenicity and safety of RotaTeq® in healthy infants in Korea ‐ NCT00166517 (PN 013). www.clinicalstudyresults.org/drugdetails/?indication_id=523&sort=c.c... 2008.
-
- NCT00166517. Immunogenicity and safety of V260 in healthy infants in Korea. clinicaltrials.gov/show/NCT00166517 (first received 14 September 2005). [Trial registration document (no data for review)]
RV5 Lawrence 2012‐CHN {published and unpublished data}
-
- Lawrence J, He S, Hille D, Shen C, Kuter B, Schodel F, et al. A study of RotaTeqTM (pentavalent rotavirus vaccine) in Chinese healthy adults, children and infants. International Journal of Infectious Diseases 2012;16(1):e307.
-
- NCT00953056. A Study of V260 in Healthy Chinese Adults, Children and Infants. clinicaltrials.gov/ct2/show/study/NCT00953056 (accessed 6 August 2009).
RV5 Levin 2017‐AF {published data only}
-
- NCT00880698. Safety and immunogenicity of a live, attenuated rotavirus (RotaTeqTM) in HIV‐1 infected and uninfected children born to HIV‐1‐infected mothers. clinicaltrials.gov/ct2/show/study/NCT00880698 (first received 14 April 2009).
-
- Uprety P, Lindsey J, Levin M, Rainwater‐Lovett K, Ziemniak C, Kaplan S, et al. Enhanced inflammation and rotavirus vaccine responses in perinatal HIV‐1 infection. Topics in Antiviral Medicine; 23rd Conference on Retroviruses and Opportunistic Infections, CROI; 2016. USA. 2016; Vol. 24(E‐1):347.
RV5 Merck[009] 2005‐USA {published and unpublished data}
-
- Merck[PN‐009]. Protocol 009 ‐ Comparison of the immunogenicity and safety of three consistency lots of RotaTeq™ in healthy infants (NCT00092456). www.clinicalstudyresults.org/drugdetails/?indication_id=523&sort=c.c... 2005.
-
- NCT00092456. Comparison of the immunogenicity and safety of three consistency lots of V260 in healthy infants. clinicaltrials.gov/ct2/show/record/NCT00092456 (first received 27 September 2004). [Trial registration document (no data for review)]
RV5 Mo 2017‐CHN {published data only}
-
- Mo Z, Mo Y, Li M, Tao J, Yang X, Kong J, et al. Efficacy and safety of a pentavalent live human‐bovine reassortant rotavirus vaccine (RV5) in healthy Chinese infants: A randomized, double‐blind, placebo‐controlled trial. Vaccine 2017;35(43):5897‐904. - PubMed
-
- NCT02062385. Efficacy, Safety, and Immunogenicity of V260 in Healthy Chinese Infants (V260‐024). clinicaltrials.gov/show/NCT02062385 (first received 13 February 2014).
RV5 Vesikari 2006a‐FIN {published and unpublished data}
-
- Vesikari T, Clark HF, Offit P, Schodel F, Dallas M, Heaton P, et al. The effect of dose and composition of a pentavalent rotavirus reassortant vaccine [RotaTeq (R)] in safety, efficacy, and immunogenicity in healthy infants. Pediatric Research 2003;53(4 Suppl):307A.
-
- Vesikari T, Clark HF, Offit PA, Dallas MJ, DiStefano DJ, Goveia MG, et al. Effects of the potency and composition of the multivalent human‐bovine (WC3) reassortant rotavirus vaccine on efficacy, safety and immunogenicity in healthy infants. Vaccine 2006;24(22):4821‐9. - PubMed
RV5 Vesikari 2006b‐INT {published and unpublished data}
-
- Chang CC, Chang MH, Lin TY, Lee HC, Hsieh WS, Lee PI. Experience of pentavalent human‐bovine reassortant rotavirus vaccine among healthy infants in Taiwan. Journal of the Formosan Medical Association 2009;108(4):280‐5. - PubMed
-
- Christie CD, Duncan ND, Thame KA, Onorato MT, Smith HD, Malcolm LG, et al. Pentavalent rotavirus vaccine in developing countries: safety and health care resource utilization. Pediatrics 2010;126(6):e1499‐506. - PubMed
-
- Dennehy PH, Goveia MG, Dallas MJ, Heaton PM. The integrated phase III safety profile of the pentavalent human‐bovine (WC3) reassortant rotavirus vaccine. International Journal of Infectious Diseases 2007;11(Suppl 2):36‐42. [No data available for review] - PubMed
-
- Goveia MG, DiNubile MJ, Dallas MJ, Heaton PM, Kuter BJ, REST Study Team. Efficacy of pentavalent human‐bovine (WC3) reassortant rotavirus vaccine based on breastfeeding frequency. Pediatric Infectious Disease Journal 2008;27(7):656‐8. - PubMed
-
- Goveia MG, Rodriguez ZM, Dallas MJ, Itzler RF, Boslego JW, Heaton PM, et al. Safety and efficacy of the pentavalent human‐bovine (WC3) reassortant rotavirus vaccine in healthy premature infants. Pediatric Infectious Disease Journal 2007;26(12):1099‐104. [No data available for review] - PubMed
RV5 Zaman 2010‐AS {published data only}
-
- Breiman RF, Zaman K, Armah G, Sow SO, Anh DD, Victor JC, et al. Analyses of health outcomes from the 5 sites participating in the Africa and Asia clinical efficacy trials of the oral pentavalent rotavirus vaccine. Vaccine 2012;30(Suppl 1):A24‐9. - PubMed
-
- Gruber JF, Hille DA, Liu GF, Kaplan SS, Nelson M, Goveia MG, et al. Heterogeneity of rotavirus vaccine efficacy among infants in developing countries. Pediatric Infectious Disease Journal 2017;36(1):72‐8. - PubMed
-
- NCT00362648. Efficacy, safety, and immunogenicity of RotaTeqTM among infants in Asia and Africa. clinicaltrials.gov/ct2/show/record/NCT00362648 (accessed 10 August 2006).
-
- Shin S, Anh DD, Zaman K, Yunus M, Mai le TP, Thiem VD, et al. Immunogenicity of the pentavalent rotavirus vaccine among infants in two developing countries in Asia, Bangladesh and Vietnam. Vaccine 2012;30(Suppl 1):A106‐13. - PubMed
-
- Zaman K, Dang DA, Victor JC, Shin S, Yunus M, Dallas MJ, et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in Asia: a randomised, double‐blind, placebo‐controlled trial. Lancet 2010;376(9741):615‐23. - PubMed
RV5 Zaman 2010‐BGD {published data only}
-
- Feller AJ, Zaman K, Lewis KD, Hossain I, Yunus M, Sack DA. Malnutrition levels among vaccinated and unvaccinated children between 2 and 3 years of age following enrollment in a randomized clinical trial with the pentavalent rotavirus vaccine (PRV) in Bangladesh. Vaccine 2012;30(Suppl 1):A101‐5. - PubMed
-
- Zaman K, Yunus M, Arifeen SE, Azim T, Faruque AS, Huq E, et al. Methodology and lessons‐learned from the efficacy clinical trial of the pentavalent rotavirus vaccine in Bangladesh. Vaccine 2012;30(Suppl 1):A94‐100. - PubMed
-
- Zaman K, Dang DA, Victor JC, Shin S, Yunus M, Dallas MJ, et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in Asia: a randomised, double‐blind, placebo‐controlled trial. Lancet 2010;376(9741):615‐23. - PubMed
RV5 Zaman 2010‐VNM {published data only}
-
- Zaman K, Dang DA, Victor JC, Shin S, Yunus M, Dallas MJ, et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in Asia: a randomised, double‐blind, placebo‐controlled trial. Lancet 2010;376(9741):615‐23. - PubMed
VAC Bhandari 2006‐IND {published data only}
-
- Bhandari N, Sharma P, Glass RI, Ray P, Greenberg H, Taneja S, et al. Safety and immunogenicity of two live attenuated human rotavirus vaccine candidates, 116E and I321, in infants: results of a randomised controlled trial. Vaccine 2006;24(31):5817‐23. - PubMed
-
- ISRCTN57452882. A double‐blind randomised placebo controlled dose escalating phase Ib/IIa study to evaluate the safety and immunogenicity of live attenuated rotavirus vaccine 116E in healthy non‐malnourished infants eight to 20 weeks of age. isrctn.com/ISRCTN57452882 (first received 26 July 2006).
-
- NCT00280111. Safety and immunogenicity study of live attenuated Indian rotavirus vaccine candidate strains 116E and I321 in infants. clinicaltrials.gov/ct2/show/NCT00280111 (first received 20 January 2006).
VAC Bhandari 2009‐IND {published data only}
-
- Bhandari N, Sharma P, Taneja S, Kumar T, Rongsen‐Chandola T, Appaiahgari MB, et al. A dose‐escalation safety and immunogenicity study of live attenuated oral rotavirus vaccine 116E in infants: a randomized, double‐blind, placebo‐controlled trial. Journal of Infectious Diseases 2009;200(3):421‐9. - PubMed
-
- ISRCTN57452882. A double‐blind randomised placebo controlled dose escalating phase Ib/IIa study to evaluate the safety and immunogenicity of live attenuated rotavirus vaccine 116E in healthy non‐malnourished infants eight to 20 weeks of age. isrctn.com/ISRCTN57452882 (first received 26 July 2006).
-
- NCT00439660. Dose escalation study to evaluate oral rotavirus vaccine 116E live attenuated in healthy infants 8 to 20 weeks old. clinicaltrials.gov/ct2/show/NCT00439660 (first received 26 February 2007).
VAC Bhandari 2014‐IND {published data only}
-
- Bhandari N, Rongsen‐Chandola T, Bavdekar A, John J, Antony K, Taneja S, et al. Efficacy of a monovalent human‐bovine (116E) rotavirus vaccine in Indian children in the second year of life. Vaccine 2014;32 Suppl 1:A110‐6. - PubMed
-
- CTRI/2010/091/000102. A phase III clinical trial to evaluate the protective efficacy of three doses of oral rotavirus vaccine (ORV) 116E. ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=1317 (first received 22 November 2010).
-
- John J, Kawade A, Rongsen‐Chandola T, Bavdekar A, Bhandari N, Taneja S, et al. Active surveillance for intussusception in a phase III efficacy trial of an oral monovalent rotavirus vaccine in India. Vaccine 2014;32 Suppl 1:A104‐9. - PubMed
-
- John TJ. Why was there no vaccine‐associated intussusception in Indian rotavirus vaccine trial?. Indian Pediatrics 2015;52(10):906. - PubMed
VAC Chandola 2017‐IND {published data only}
-
- CTRI/2014/05/004592. A phase III study to evaluate the non‐interference in the immune response of rotavac to childhood vaccines and to assess the clinical lot consistency. ctri.nic.in/Clinicaltrials/pdf_generate.php?trialid=5870&EncHid=&... (first received 13 May 2014).
References to studies excluded from this review
OTHER Armah 2013 {published data only}
-
- Armah GE, Kapikian AZ, Vesikari T, Cunliffe N, Jacobson RM, Burlington DB, et al. Efficacy, immunogenicity, and safety of two doses of a tetravalent rotavirus vaccine RRV‐TV in Ghana with the first dose administered during the neonatal period. Journal of Infectious Diseases 2013;208(3):423‐31. - PMC - PubMed
OTHER Bines 2015 {published data only}
-
- ACTRN12611001212943. RV3‐BB rotavirus vaccine phase IIa clinical trial of immunogenicity and safety. anzctr.org.au/ACTRN12611001212943.aspx (first received 24 November 2011).
-
- Bines JE, Danchin M, Jackson P, Handley A, Watts E, Lee KJ, et al. RV3 Rotavirus Vaccine Program. Safety and immunogenicity of RV3‐BB human neonatal rotavirus vaccine administered at birth or in infancy: a randomised, double‐blind, placebo‐controlled trial. Lancet Infectious Diseases 2015;15(12):1389‐97. - PubMed
OTHER Bines 2018 {published data only}
OTHER Bucardo 2018 {published data only}
OTHER Bucher 2012 {published data only}
-
- Bucher A, Rivara G, Briceno D, Huicho L. [Use of a rapid rotavirus test in prescription of antibiotics in acute diarrhea in pediatrics: an observational, randomized, controlled study]. Revista de Gastroenterologia del Peru 2012;32(1):11‐5. - PubMed
OTHER Chatterjee 2012 {published data only}
-
- Chatterjee A, O'Keefe C, Varman M, Klein NP, Luber S, Tomovici A, et al. Comparative immunogenicity and safety of different multivalent component pertussis vaccine formulations and a 5‐component acellular pertussis vaccine in infants and toddlers: a randomized, controlled, open‐label, multicenter study. Vaccine 2012; Vol. 30, issue 23:3360‐8. - PubMed
OTHER Cowley 2017 {published data only}
OTHER CTRI/2009/091/000821 {published data only}
-
- CTRI‐2009‐091‐000821. A randomized, double‐blind, placebo controlled study to assess the safety and tolerability of RotaVac vaccine (live attenuated bovine‐human (UK) reassortant pentavalent rotavirus vaccine). ctri.in/Clinicaltrials/ViewTrial.jsp?trialno=1302 (first received 15 October 2009).
OTHER Dang 2012 {published data only}
-
- Dang DA, Nguyen VT, Vu DT, Nguyen TH, Nguyen DM, Yuhuan W. A dose‐escalation safety and immunogenicity study of a new live attenuated human rotavirus vaccine (Rotavin‐M1) in Vietnamese children. Vaccine 2012;30(Suppl 1):A114‐21. - PubMed
-
- NCT01377571. A dose‐escalating study to evaluate the immunogenicity and safety of rotavin‐M1 vaccine in healthy infants. clinicaltrials.gov/ct2/show/NCT01377571 (first received 21 June 2011).
OTHER de Palma 2010 {published data only}
OTHER Dickson 2017 {published data only}
-
- Dickson I. Diarrhoea: low‐cost rotavirus vaccine shows efficacy in Niger. Nature Reviews. Gastroenterology & Hepatology 2017;14(5):260. - PubMed
OTHER Diness 2010 {published data only}
-
- Diness BR, Christoffersen D, Pedersen UB, Rodrigues A, Fischer TK, Andersen A, et al. The effect of high‐dose vitamin A supplementation given with bacille Calmette‐Guerin vaccine at birth on infant rotavirus infection and diarrhea: a randomized prospective study from Guinea‐Bissau. Journal of Infectious Disease 2010;202(Suppl):S243‐51. - PubMed
OTHER Dutta 2011 {published data only}
-
- Dutta P, Mitra U, Dutta S, Rajendran K, Saha TK, Chatterjee MK. Randomised controlled clinical trial of Lactobacillus sporogenes (Bacillus coagulans), used as probiotic in clinical practice, on acute watery diarrhoea in children. Tropical Medicine and International Health 2011;16(5):555‐61. - PubMed
OTHER Ella 2018 {published data only}
-
- CTRI/2014/04/004548. A phase IV immunogenicity and safety study of BBILs oral rotavirus vaccine 116E (ROTAVAC). ctri.nic.in/Clinicaltrials/pdf_generate.php?trialid=7937&EncHid=&... (first receiveded 17 April 2014).
OTHER Friedrich 2017 {published data only}
-
- Friedrich MJ. Freeze‐dried rotavirus vaccine shows promise. JAMA 2017;317(19):1941. - PubMed
OTHER Gagneur 2011 {published data only}
-
- Gagneur A, Nowak E, Lemaitre T, Segura JF, Delaperriere N, Abalea L, et al. IVANHOE investigators. Impact of rotavirus vaccination on hospitalizations for rotavirus diarrhea: The IVANHOE study. Vaccine 2011;29(21):3753‐9. - PubMed
OTHER Groome 2017 {published data only}
OTHER Hiramatsu 2018 {published data only}
-
- Hiramatsu H, Suzuki R, Nagatani A, Boda H, Miyata M, Hattori F, et al. Rotavirus vaccination can be performed without viral dissemination in the neonatal intensive care unit. Journal of Infectious Diseases 2018;217(4):589‐96. - PubMed
OTHER Isanaka 2017‐NER {published data only}
-
- Isanaka S, Djibo A, Grais RF. Heat‐stable oral rotavirus vaccine. New England Journal of Medicine 2017;377(3):302. - PubMed
-
- Isanaka S, Guindo O, Langendorf C, Grais R, ROSE Trial Study Team. Efficacy and safety of a low‐cost, heat‐stable oral roatvirus vaccine agains severe rotavirus gastroenteritis in Niger. American Journal of Tropical Medicine and Hygiene 2017;95:242.
-
- Isanaka S, Guindo O, Langendorf C, Matar Seck A, Plikaytis BD, Sayinzoga‐Makombe N, et al. Efficacy of a low‐cost, heat‐stable oral rotavirus vaccine in Niger. New England Journal of Medicine 2017;376(12):1121‐30. - PubMed
-
- NCT02145000. Efficacy and safety of a pentavalent rotavirus vaccine (BRV‐PV) against severe rotavirus gastroenteritis in Niger (ROSE). clinicaltrials.gov/ct2/show/NCT02145000 (accessed 22 May 2014).
OTHER Kempe 2007 {published data only}
-
- Kempe A, Daley MF, Parashar UD, Crane LA, Beaty BL, Stokley S, et al. Will pediatricians adopt the new rotavirus vaccine?. Pediatrics 2007;119(1):1‐10. - PubMed
OTHER Kulkarni 2017 {published data only}
-
- CTRI/2013/05/003667. A clinical trial to study the effect and safety of Rotavirus Vaccine against Severe Rotavirus Gastroenteritis in healthy Indian Infants. ctri.nic.in/Clinicaltrials/pdf_generate.php?trialid=5518&EncHid=&... (first received 23 May 2013).
-
- NCT02133690. A clinical trial to study the effect and safety of rotavirus vaccine against severe rotavirus gastroenteritis in healthy Indian infants. clinicaltrials.gov/ct2/show/NCT02133690 (first received 8 May 2014).
-
- Zade JK, Kulkarni PS, Desai SA, Sabale RN, Naik SP, Dhere RM. Bovine rotavirus pentavalent vaccine development in India. Vaccine 2014;32(Suppl 1):A124‐8. - PubMed
OTHER Muhsen 2010 {published data only}
-
- Muhsen K, Shulman L, Kasem E, Rubinstein U, Shachter J, Kremer A, et al. Effectiveness of rotavirus vaccines for prevention of rotavirus gastroenteritis‐associated hospitalizations in Israel: a case‐control study. Human Vaccines 2010;6(6):450‐4. - PubMed
OTHER NCT00981669 {published data only}
-
- NCT00981669. Evaluation of rotavirus vaccine produced by Butantan Institute. Phase I ‐ safety, tolerability and immunogenicity evaluation. clinicaltrials.gov/show/NCT00981669 (first received 22 September 2009).
OTHER NCT01195844 {published data only}
-
- NCT01195844. Rotavirus gastroenteritis in children less than 5 years‐old. Surveillance performed in hospitals from four Brazilian regions. clinicaltrials.gov/show/NCT01195844 (first received 6 September 2010).
OTHER NCT01236066 {published data only}
-
- NCT01236066. A study on the impact of rotavirus vaccination on hospitalisations for rotavirus gastroenteritis in children aged <5 years in Australia. clinicaltrials.gov/show/NCT01236066 (first received 8 November 2010).
OTHER NCT01375907 {published data only}
-
- NCT01375907. A Phase 1 study to evaluate safety and reactogenicity of a Vietnamese rotavirus vaccine (Rotavin‐M1 at 10e6.3FFU/Dose) among healthy adults in Vietnam. clinicaltrials.gov/show/NCT01375907 (first received 17 June 2011).
OTHER NCT01571505 {published data only}
-
- NCT01571505. Exploration of the biologic basis for underperformance of oral polio and rotavirus vaccines in India (PROVIDE). clinicaltrials.gov/show/NCT01571505 (first received 5 April 2012).
OTHER Rivera 2011 {published data only}
-
- Rivera L, Peña LM, Stainier I, Gillard P, Cheuvart B, Smolenov I, et al. Horizontal transmission of a human rotavirus vaccine strain‐‐a randomized, placebo‐controlled study in twins. Vaccine 2011;29(51):9508‐13. - PubMed
OTHER Thyagarajan 2011 {published data only}
-
- Thyagarajan V, Glass R, Rodgers K, Quinlan S, Holick CN, Rosillon D, et al. Validity of current procedural terminology codes for rotavirus vaccination in two commercially‐insured US populations. Pharmacoepidemiology and Drug Safety 2011;20:S242. - PubMed
OTHER Yin 2017 {published data only}
OTHER Zade 2014a‐IND {published data only}
-
- Zade JK, Kulkarni PS, Desai SA, Sabale RN, Naik SP, Dhere RM. Bovine rotavirus pentavalent vaccine development in India. Vaccine 2014 Aug 11;32(Suppl 1):A124‐8. - PubMed
OTHER Zade 2014b‐IND {published data only}
-
- CTRI/2010/091/003064. A randomized, double‐blind, placebo controlled study to assess safety and tolerability of RotaVac vaccine (Live Attenuated Bovine‐Human (UK) Reassortant Pentavalent Rotavirus Vaccine). ctri.nic.in/Clinicaltrials/pdf_generate.php?trialid=2458&EncHid=&... (first received 04 January 2011).
-
- Zade JK, Kulkarni PS, Desai SA, Sabale RN, Naik SP, Dhere RM. Bovine rotavirus pentavalent vaccine development in India. Vaccine 2014;32(Suppl 1):A124‐8. - PubMed
RV1 / RV5 Libster {published data only}
-
- NCT01266850. Safety and Immunogenicity of Sequential Rotavirus Vaccine Schedule. clinicaltrials.gov/ct2/show/NCT01266850 (first received 24 December 2010).
RV1 Ali 2014 {published data only}
-
- Ali SA, Kazi AM, Cortese MM, Fleming JA, Parashar UD, Jiang B, et al. Impact of different dosing schedules on the immunogenicity of the human rotavirus vaccine in infants in Pakistan: a randomized trial. Journal of Infectious Diseases 2014;210(11):1772‐9. - PubMed
-
- NCT01199874. The immunogenicity of rotavirus vaccine under different age schedules and the impact of withholding breast feeding around the time of vaccination on the immunogenicity of Rotarix vaccine. clinicaltrials.gov/show/NCT01199874 (first received 13 September 2010).
RV1 Armah 2016 {published data only}
-
- NCT01575197. Evaluation of the human rotavirus vaccine when given at varying schedules in rural Ghana. clinicaltrials.gov/show/NCT01575197 (first received 11 April 2012).
RV1 Buyse 2014 {published data only}
RV1 Correia 2010 {published data only}
-
- Correia JB, Patel MM, Nakagomi O, Montenegro FM, Germano EM, Correia NB, et al. Effectiveness of monovalent rotavirus vaccine (Rotarix) against severe diarrhea caused by serotypically unrelated G2P[4] strains in Brazil. Journal of Infectious Diseases 2010;201(3):363‐9. - PubMed
RV1 CTRI/2012/02/002454 {published data only}
-
- CTRI/2012/02/002454. Comparison of immunogenicity of a 3 dose and a 5 dose schedule of oral rotavirus vaccine in south Indian infants. ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=4262 (first received 28 May 2012).
RV1 Dennehy 2008 {published data only}
-
- Dennehy PH, Bertrand HR, Silas PE, Damaso S, Friedland LR, Abu‐Elyazeed R. Coadministration of RIX4414 oral human rotavirus vaccine does not impact the immune response to antigens contained in routine infant vaccines in the United States. Pediatrics 2008;122(5):e1062‐6. - PubMed
-
- NCT00334607. Assess the immunogenicity of 3 doses of Pediarix®, Prevnar® & ActHIB® given to healthy infants when administered with GSK Biologicals’ 2 dose oral live attenuated human rotavirus vaccine given during the same vaccination visit or separately. clinicaltrials.gov/show/NCT00334607 (first received 8 June 2006).
RV1 Emperador 2016 {published data only}
RV1 GSK[107077‐057] 2008 {published data only}
-
- GlaxoSmithKline[107077‐057]. A phase III, open, randomized study to assess the immunogenicity, reactogenicity and safety of two different formulations of GlaxoSmithKline (GSK) Biologicals’ live attenuated human rotavirus (HRV) vaccine, given as a two‐dose primary vaccination, in healthy infants previously uninfected with HRV. ctr.gsk.co.uk/Summary/Vaccine_Rotavirus/III_107077.pdf (accessed before 09 October 2018).
RV1 GSK[107876‐061] 2008 {published data only}
-
- GlaxoSmithKline[107876‐061]. A phase III, randomised study to evaluate the clinical consistency in terms of immunogenicity and reactogenicity of three production lots of the liquid formulation of GlaxoSmithKline (GSK) Biologicals’ oral live attenuated human rotavirus (HRV) vaccine and to evaluate the liquid formulation as compared to the lyophilised formulation of the HRV vaccine in terms of immunogenicity, reactogenicity and safety when administered as a two‐dose primary vaccination in healthy infants previously uninfected with human rotavirus. ctr.gsk.co.uk/Summary/Vaccine_Rotavirus/III_107876.pdf (accessed before 09 October 2018).
RV1 GSK[444563‐020] 2007 {published data only}
-
- GlaxoSmithKline[444563‐020]. A phase II, double‐blind randomised, placebo controlled clinical dose‐range study to assess the immunogenicity and reactogenicity of an investigational vaccination regimen, and to assess the immunogenicity of OPV orally co‐administered to healthy infants at 2, 4 and 6 months of age. ctr.gsk.co.uk/Summary/Vaccine_Rotavirus/II_444563_020.pdf (accessed before 09 October 2018).
RV1 Herrera 2013 {published data only}
RV1 Kazi 2017 {published data only}
-
- Kazi AM, Cortese MM, Yu Y, Lopman B, Morrow AL, Fleming JA, et al. Secretor and salivary ABO blood group antigen status predict rotavirus vaccine take in infants. Journal of Infectious Diseases 2017;215(5):786‐9. - PubMed
RV1 Kompithra 2014 {published data only}
-
- Kompithra RZ, Paul A, Manoharan D, Babji S, Sarkar R, Mathew LG, et al. Immunogenicity of a three dose and five dose oral human rotavirus vaccine (RIX4414) schedule in south Indian infants. Vaccine 2014;32 Suppl 1:A129‐33. - PubMed
RV1 Lazarus 2017 {published data only}
-
- NCT01616693. Zinc and/or probiotic supplementation of rotavirus and oral polio virus vaccines. clinicaltrials.gov/ct2/show/NCT01616693 (first received 12 June 2012).
RV1 Lu 2013 {published data only}
-
- Lu CY, Chang LY, Shao PL, Suryakiran PV, Han HH, Huang LM. Immunogenicity, reactogenicity, and safety of a human rotavirus vaccine, Rotarix, in Taiwanese infants who received a dose of hepatitis B immunoglobulin after birth. Journal of the Formosan Medical Association 2013;112(9):574‐7. - PubMed
RV1 NCT00353366 {published data only}
-
- NCT00353366. A study to evaluate the safety & reactogenicity of GSK Bio's live attenuated oral HRV vaccine, Rotarix when administered according to prescribing information, in Filipino subjects aged between 6 weeks & 14 weeks at first vaccination. clinicaltrials.gov/show/NCT00353366 (first received 18 July 2006).
RV1 NCT00382772 2008 {published data only}
-
- NCT00382772. Study to evaluate clinical consistency of the liquid formulation of GSK Biologicals' HRV vaccine and to evaluate liquid formulation compared to lyophilised formulation of the HRV vaccine administered as a two‐dose primary vaccination. clinicaltrials.gov/ct2/show/record/NCT00382772 (first received 02 October 2006).
RV1 NCT00653198 {published data only}
-
- NCT00653198. Hospital‐based, case‐control study to assess the vaccine effectiveness of Rotarix™ against rotavirus severe gastroenteritis (RV SGE) among hospitalised children born after 1 March 2006 and at least 12 weeks of age, in Panama. clinicaltrials.gov/show/NCT00653198 (first received 4 April 2008).
RV1 NCT00655187 {published data only}
-
- NCT00655187. Hospital‐based, case‐control study to assess the vaccine effectiveness of Rotarix™ against rotavirus severe gastroenteritis (RV SGE) among hospitalised children < 5 years of age in KK Hospital, Singapore. clinicaltrials.gov/show/NCT00655187 (first received 9 April 2008).
RV1 NCT01162590 {published data only}
-
- NCT01162590. Reactogenicity and safety of a single dose of GlaxoSmithKline (GSK) Biologicals' human rotavirus (HRV) vaccine (444563) in healthy adults. clinicaltrials.gov/show/NCT01162590 (first received 14 July 2010).
RV1 NCT01177826 {published data only}
-
- NCT01177826. Case‐control study to evaluate the vaccine effectiveness of GlaxoSmithKline (GSK) Biologicals' Live Attenuated Human Rotavirus (HRV) Vaccine (Rotarix™) against community‐acquired rotavirus severe gastroenteritis (RV SGE) among hospitalised children born after 1 October 2006, in Belgium. clinicaltrials.gov/show/NCT01177826 (first received 9 August 2010).
RV1 NCT01273077 {published data only}
-
- NCT01273077. Evaluation of universal rotavirus vaccination program. clinicaltrials.gov/show/NCT01273077 (first received 10 January 2011).
RV1 NCT01339221 {published data only}
-
- NCT01339221. Epidemiological, observational, post marketing study of the genetic stability of GSK Biologicals' Rotavirus Vaccine (Rotarix™) in children <5 years of age diagnosed with severe gastroenteritis, in Belgium. clinicaltrials.gov/show/NCT01339221 (first received 20 April 2011).
RV1 Plosker 2011 {published data only}
-
- Plosker GL. Rotavirus vaccine RIX4414 (Rotarix): A pharmacoeconomic review of its use in the prevention of rotavirus gastroenteritis in developed countries. Pharmacoeconomics 2011;29(5):439‐54. - PubMed
RV1 Ramani 2016 {published data only}
-
- Ramani S, Mamani N, Villena R, Bandyopadhyay AS, Gast C, Sato A, et al. Rotavirus serum IgA immune response in children receiving rotarix coadministered with bOPV or IPV. Pediatric Infectious Disease Journal 2016;35(10):1137‐9. - PubMed
RV1 Rojas 2007 {published data only}
-
- Rojas OL, Caicedo L, Guzman C, Rodriguez LS, Castaneda J, Uribe L, et al. Evaluation of circulating intestinally committed memory B cells in children vaccinated with attenuated human rotavirus vaccine. Viral Immunology 2007;20(2):300‐11. - PubMed
RV1 Rongsen‐Chandola 2014 {published data only}
-
- Rongsen‐Chandola T, Strand TA, Goyal N, Flem E, Rathore SS, Arya A, et al. Effect of withholding breastfeeding on the immune response to a live oral rotavirus vaccine in North Indian infants. Vaccine 2014 Aug 11;32(Suppl 1):A134‐9. - PubMed
RV1 Suryakiran 2011 {published data only}
-
- Suryakiran PV, Vinals C, Vanfraechem K, Han HH, Guerra Y, Buyse H. The human rotavirus vaccine rix4414 in infants: an integrated safety summary (ISS). Acta Paediatrica, International Journal of Paediatrics 2011;100:56‐7.
RV1 Taddio 2015 {published data only}
-
- Taddio A, Flanders D, Weinberg E, Lamba S, Vyas C, Ilersich AF, et al. A randomized trial of rotavirus vaccine versus sucrose solution for vaccine injection pain. Vaccine 2015;33(25):2939‐43. - PubMed
RV1 Zaman 2016 {published data only}
-
- NCT01700621. Coadministration of measles‐rubella and rotavirus vaccines. clinicaltrials.gov/ct2/show/NCT01700621 (first received on 4 October 2012).
RV5 / BRV‐TV Saluja 2017 {published data only}
-
- CTRI/2014/08/004893. A study to evaluate immune non‐inferiority and safety of tetravalent Rotavirus Vaccine (BRV‐TV) in comparison to licensed vaccine (RotaTeq) in healthy infants. ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=9950&EncHid=&us... (first received 20 August 2014).
-
- Saluja T, Palkar S, Misra P, Gupta M, Venugopal P, Sood AK, et al. Live attenuated tetravalent (G1‐G4) bovine‐human reassortant rotavirus vaccine (BRV‐TV): randomized, controlled phase III study in Indian infants. Vaccine 2017;35(28):3575‐81. - PubMed
RV5 ACTRN12611000559910 {published data only}
-
- ACTRN 12611000559910. An observational, cross sectional, cohort study to assess the impact of rotavirus vaccine introduction on severe gastroenteritis in South Australian children. anzctr.org.au/ACTRN12611000559910.aspx (first received 31 May 2011).
RV5 Ciarlet 2008 {published data only}
-
- Ciarlet M, Sani‐Grosso R, Yuan G, Liu GF, Heaton PM, Gottesdiener KM, et al. Concomitant use of the oral pentavalent human‐bovine reassortant rotavirus vaccine and oral poliovirus vaccine. Pediatric Infectious Disease Journal 2008;27(10):874‐80. - PubMed
RV5 El Khoury 2011 {published data only}
-
- Khoury AC, Mast TC, Ciarlet M, Markson L, Goveia MG, Munford V, et al. Projecting the effectiveness of RotaTeq against rotavirus‐related hospitalisations in Brazil. Memorias do Instituto Oswaldo Cruz 2011;106(5):541‐5. - PubMed
RV5 El Khoury 2011a {published data only}
RV5 Martinon‐Torres 2017 {published data only}
-
- Martinon‐Torres F, Greenberg D, Varman M, Killar JA, Hille D, Strable EL, et al. Safety, tolerability and immunogenicity of pentavalent rotavirus vaccine manufactured by a modified process. Pediatric Infectious Disease Journal 2017;36(4):417‐22. - PubMed
RV5 McGrath 2014 {published data only}
RV5 NCT00130832 2010 {published data only}
-
- NCT00130832. Concomitant use and staggered use of vaccine and oral poliovirus (OPV) in healthy infants. clinicaltrials.gov/ct2/show/results/NCT00130832 (first received 16 August 2005).
RV5 NCT00496054 {published data only}
-
- NCT00496054. Evaluation of safety, tolerability and immunogenicity of vaccination with Rotateq (V260) in healthy infants in India. clinicaltrials.gov/show/NCT00496054 (first received 4 July 2007).
RV5 NCT01926015 {published data only}
-
- NCT01926015. Immunogenicity and safety of concomitant administration of RotaTeq™ (V260) and the diphtheria, tetanus, pertussis and inactivated poliovirus vaccine (DTP‐IPV) in healthy Japanese infants (V260‐060). clinicaltrials.gov/ct2/show/NCT01926015 (first received on 20 August 2013).
RV5 Saleh 2018 {published data only}
-
- Saleh E, Eichner B, Clark DW, Gagliano ME, Troutman JM, Harrington L, et al. Open‐label pilot study to compare the safety and immunogenicity of pentavalent rotavirus vaccine (RV5) administered on an early alternative dosing schedule with those of RV5 administered on the recommended standard schedule. Journal of the Pediatric Infectious Diseases Society 2018;7(1):82‐5. - PMC - PubMed
RV5 Tugcu 2009 {published data only}
-
- Tugcu U, Sahin F, Bozdayi G, Aksakal FN, Alp G, Rota S, et al. Clinical efficacy of rotavirus vaccine in Turkish infants. Journal of Clinical Virology 2009; Vol. 46, issue Suppl:S15–S61.
RV5 Uprety 2017 {published data only}
-
- Uprety P, Lindsey JC, Levin MJ, Rainwater‐Lovett K, Ziemniak C, Bwakura‐Dangarembizix M, et al. Inflammation and immune activation in antiretroviral‐treated human immunodeficiency virus type 1‐infected African infants and rotavirus vaccine responses. Journal of Infectious Diseases 2017;215(6):928‐32. - PMC - PubMed
RV5 Vesikari 2011 {published data only}
-
- * Vesikari T, Karvonen A, Borrow R, Kitchin N, Baudin M, Thomas S, et al. Results from a randomized clinical trial of coadministration of RotaTeq, a pentavalent rotavirus vaccine, and NeisVac‐C, a meningococcal serogroup C conjugate vaccine. Clinical Vaccine Immunology 2011 May;18(5):878‐84. - PMC - PubMed
-
- NCT00443846. An open‐label, randomised, comparative, multi‐centre study of the immunogenicity and safety of the concomitant use of a live pentavalent rotavirus vaccine (RotaTeq®) and a meningococcal Group C conjugate (MCC) vaccine in healthy infants. clinicaltrials.gov/ct2/show/record/NCT00443846 (first received 6 March 2007).
RV5 Weinberg 2017 {published data only}
References to ongoing studies
OTHER ACTRN12610000525088 {published data only}
-
- ACTRN12610000525088. A Phase 1 double‐blind, randomized study to compare the safety, tolerability and immunogenicity of oral RV3‐BB rotavirus vaccine and placebo in infants, children and male adults. anzctr.org.au/ACTRN12610000525088.aspx (first received 22 June 2010).
OTHER CTRI/2015/07/006034 {published data only}
-
- CTRI/2015/07/006034. Clinical trial on rotavirus vaccine to check consistency of different lots of vaccines manufactured and to check vaccine interference with other childhood vaccines given under universal immunization program in India. ctri.nic.in/Clinicaltrials/pdf_generate.php?trialid=10177&EncHid=&am... (first received 20 July 2015).
OTHER CTRI/2015/12/006428 {published data only}
-
- CTRI/2015/12/006428. Randomized open label study to compare immunogenicity and safety of ROTAVAC® and ROTARIX® rotavirus vaccine. ctri.nic.in/Clinicaltrials/pdf_generate.php?trialid=13227&EncHid=&am... (first received 8 December 2015).
OTHER NCT01061658 {published data only}
-
- NCT01061658. Phase I/II, randomized, double‐blind, placebo‐controlled, dosage selection (10e5.5 or 10e6.25 FFU of each constituent serotype per 0.5 mL) study to evaluate the safety, tolerability, and immunogenicity of a 3‐dose Series of live attenuated tetravalent (G1‐G4) bovine‐human reassortant rotavirus vaccine [BRV‐TV] administered to healthy Indian infants. clinicaltrials.gov/show/NCT01061658 (accessed 3 February 2010).
OTHER NCT02153866 {published data only}
-
- NCT02153866. The safety and immunogenicity study of rotavirus vaccine simultaneously vaccinated with MR or MMR vaccine. clinicaltrials.gov/ct2/show/NCT02153866 (first received 3 June 2014).
OTHER NCT02193061 {published data only}
-
- NCT02193061. Randomized, controlled single‐blind clinical study to assess vaccine interchangeability between RV5 and RV1 using seven combined anti‐rotavirus prevention programs. clinicaltrials.gov/ct2/show/NCT02193061 (first received 17 July 2014).
OTHER NCT02542462 {published data only}
-
- NCT02542462. Potential mechanisms for intussusception after rotavirus vaccine‐pilot study. clinicaltrials.gov/ct2/show/NCT02542462 (first received 7 September 2015).
OTHER NCT02646891 {published data only}
-
- NCT02646891. Safety and immunogenicity study of trivalent P2‐VP8 subunit rotavirus vaccine in adults, toddlers and infants. clinicaltrials.gov/ct2/show/NCT02646891 (first received 6 January 2016).
OTHER NCT02847026 {published data only}
-
- NCT02847026. Fractional inactivated poliovirus vaccine booster and rotavirus study (fIPV). clinicaltrials.gov/ct2/show/NCT02847026 (accessed 24 April 2018) (first received 2 July 2016).
OTHER NCT03462108 {published data only}
-
- NCT03462108. Safety and immunogenicity of Rotavirus (Bio Farma) vaccine in adults, children & neonates. clinicaltrials.gov/ct2/show/NCT03462108 (first received 12 March 2018).
OTHER NCT03483116 {published data only}
-
- NCT03483116. A Phase II randomized, double blind, parallel group dose‐ranging study of oral RV3‐BB rotavirus vaccine. clinicaltrials.gov/ct2/show/NCT03483116 (first received 30 March 2018).
RV1 ISRCTN86632774 {published data only}
-
- ISRCTN86632774. A phase II, double blind randomised, placebo controlled study to assess the safety reactogenicity and immunogenicity of three doses of GSK Biologicals (South Africa). controlled‐trials.com/ISRCTN86632774 (first received 25 November 2005).
RV1 NCT02941107 {published data only}
-
- NCT02941107. Optimising rotavirus vaccine in Aboriginal children. clinicaltrials.gov/ct2/show/NCT02941107 (first received 21 October 2016).
RV1 Tatochenko 2008 {published data only}
-
- Tatochenko VK, Romanenko VV, Kharit SM, Smolenov I, LeFevre I, Clercq N, et al. Co‐administration of a human rotavirus vaccine Rix4414 with DTPw‐HBv vaccines: immunogenicity and reactogenicity in healthy infants. 13th International Congress on Infectious Diseases, Kuala Lumpur, Malaysia; June 19–22. 2008.
RV5 NCT02728869 {published data only}
-
- NCT02728869. Safety, reactogenicity and immunogenicity of heat‐stable rotavirus vaccine (HSRV) in adults and infants. clinicaltrials.gov/ct2/show/NCT02728869 (first received 5 April 2016).
Additional references
Bar‐Zeev 2015
Bar‐Zeev 2018
Bines 2005
-
- Bines JE. Rotavirus vaccines and intussusception risk. Current Opinion in Gastroenterology 2005;21(1):20‐5. - PubMed
Buttery 2011
-
- Buttery JP, Danchin MH, Lee KJ, Carlin JB, McIntyre PB, Elliott EJ, et al. Intussusception following rotavirus vaccine administration: Post‐marketing surveillance in the National Immunization Program in Australia. Vaccine 2011;29(16):3061‐66. - PubMed
Buyse 2014
Bányai 2012
-
- Bányai K, László B, Duque J, Steele AD, Nelson EA, Gentsch JR, et al. Systematic review of regional and temporal trends in global rotavirus strain diversity in the prerotavirus vaccine era: insights for understanding the impact of rotavirus vaccination programs. Vaccine 2012;30(Suppl 1):A122‐30. - PubMed
Calles 2010
-
- Calles NR, Schutze GE. Immunizations for children with HIV. Module for HIV Curriculum available from: bipai.org/sites/bipai/files/20‐Immunizations.pdf (accessed 25 Oct 2018).
Carlin 2013
-
- Carlin JB, Macartney KK, Lee KJ, Quinn HE, Buttery J, Lopert R, et al. Intussusception risk and disease prevention associated with rotavirus vaccines in Australia's National Immunization Program. Clinical Infectious Diseases 2013;57:1427‐34. - PubMed
CDC 2010
-
- Advisory Committee on Immunization Practices, Department of Health and Human Services, Center for Disease Control and Prevention. Summary Report, October 27‐28, 2010. cdc.gov/vaccines/recs/acip/downloads/min‐archive/min‐oct10.pdf (URL no longer available) (accessed 02 March 2012).
CDC‐ASIP 1999
-
- Advisory Committee on Immunization Practices. Rotavirus vaccine for the prevention of rotavirus gastroenteritis among children. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR. Recommendations and Reports 1999;48(RR‐2):1‐20. - PubMed
Crawford 2017
Cunliffe 2016
-
- Cunliffe NA, Kang G. Can changes to scheduling enhance the performance of rotavirus vaccines in low‐income countries?. Journal of Infectious Diseases 2016;213(11):1673‐5. - PubMed
Czerkinsky 2015
Dellepiane 2015
Dennehy 2008
-
- Dennehy PH, Brady RC, Halperin SA, Ward RL, Alvey JC, Fischer FH Jr, et al. North American Human Rotavirus Vaccine Study Group. Comparative evaluation of safety and immunogenicity of two dosages of an oral live attenuated human rotavirus vaccine. Pediatric Infectious Disease Journal 2005;24(6):481‐8. - PubMed
Do Carmo 2011
Dutta 2017
-
- Dutta AK. Rotavirus vaccination in India ‐ need for surveillance of intussusception. Indian Journal of Pediatrics 2017;84(2):95‐6. - PubMed
EMA 2011
-
- European Medicines Agency. Summary of Product Characteristics: Rotarix. ema.europa.eu/docs/en_GB/document_library/EPAR_‐_Product_Information/hum... (accessed November 2011).
Escolano 2011
-
- Escolano S, Farrington CP, Hill C, Tubert‐Bitter P. Intussusception after rotavirus vaccination‐‐spontaneous reports. New England Journal of Medicine 2011;365(22):2139. - PubMed
Escolano 2015
-
- Escolano S, Hill C, Tubert‐Bitter P. Intussusception risk after RotaTeq vaccination: evaluation from worldwide spontaneous reporting data using a self‐controlled case series approach. Vaccine 2015;33(8):1017‐20. - PubMed
Fischer Walker 2011
Gladstone 2011
GRADE 2004 [Computer program]
-
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed before 09 October 2018. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Groome 2014
-
- Groome MJ, Page N, Cortese MM, Moyes J, Zar HJ, Kapongo CN, et al. Effectiveness of monovalent human rotavirus vaccine against admission to hospital for acute rotavirus diarrhoea in South African children: a case‐control study. Lancet Infectious Diseases 2014;14(11):1096‐1104. - PubMed
Higgins 2003
Higgins 2011
-
- Higgins JP, Deeks JJ, Altman DG, editor(s). Chapter 16: Special topics in statistics. In: Higgins JP, Green S, editor(s), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org.
Higgins 2017
-
- Higgins JP, Altman DG, Sterne JAC, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017) Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Hungerford 2015
Hungerford 2018
Jonesteller 2017
-
- Jonesteller CL, Burnett E, Yen C, Tate JE, Parashar UD. Effectiveness of rotavirus vaccination: a systematic review of the first decade of global postlicensure data, 2006‐2016. Clinical Infectious Diseases 2017;65(5):840‐50. - PubMed
Lamberti 2016
-
- Lamberti LM, Ashraf S, Walker CL, Black RE. A systematic review of the effect of rotavirus vaccination on diarrhea outcomes among children younger than 5 years. Pediatric Infectious Disease Journal 2016;35(9):992‐8. - PubMed
Lefebvre 2011
-
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org.
Levine 2010
Linhares 2008
-
- Linhares AC, Velázquez FR, Pérez‐Schael I, Sáez‐Llorens X, Abate H, Espinoza F, et al. Human Rotavirus Vaccine Study Group. Efficacy and safety of an oral live attenuated human rotavirus vaccine against rotavirus gastroenteritis during the first 2 years of life in Latin American infants: a randomised, double‐blind, placebo‐controlled phase III study. Lancet 2008;371(9619):1181‐9. - PubMed
Merck 2008
-
- Merck. WHO list of vaccines for purchase by UN agencies as of November 2008. www.who.int/immunization_standards/vaccine_quality/pq_suppliers/en/index.... Press release (accessed 21 November 2008).
Merck 2012
-
- Merck, Co. Inc. Quality details for 9 RV5 included trials. Correspondence, 12 March 2012.
Murphy 2001
-
- Murphy TV, Gargiullo PM, Massoudi MS, Nelson DB, Jumaan AO, Okoro CA, et al. Intussusception among infants given an oral rotavirus vaccine. New England Journal of Medicine 2001;344(8):564‐72. - PubMed
Parashar 2006a
-
- Parashar UD, Glass RI. Public health. Progress toward rotavirus vaccines. Science 2006;312(5775):851‐2. - PubMed
Parashar 2006b
-
- Parashar UD, Alexander JP, Glass RI, Advisory Committee on Immunization Practices (ACIP), Centers for Disease Control and Prevention (CDC). Prevention of rotavirus gastroenteritis among infants and children. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR. Recommendations and Reports 2006;55(RR‐12):1‐13. - PubMed
Patel 2011
-
- Patel MM, Lopez‐Collada VR, Bulhoes MM, Oliveira LH, Marquez AB, Flannery B, et al. Intussusception risk and health benefits of rotavirus vaccination in Mexico and Brazil. New England Journal of Medicine 2011;364(24):2283‐92. - PubMed
Patel 2012
PATH 2016
-
- PATH. Country National Immunization Program (NIP) Introductions of Rotavirus Vaccine. www.vaccineresources.org/files/PATH‐Country‐Introduction‐Table‐EN‐2016.0... (accessed 27 July 2018).
Pinto 2016
-
- Pinto MV, Bihari S, Snape MD. Immunisation of the immunocompromised child. Journal of Infection 2016;72 Suppl:S13‐22. - PubMed
RevMan 2014 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Richardson 2010
-
- Richardson V, Hernandez‐Pichardo J, Quintanar‐Solares M, Esparza‐Aguilar M, Johnson B, Gomez‐Altamirano CM, et al. Effect of rotavirus vaccination on death from childhood diarrhea in Mexico. New England Journal of Medicine 2010;362(4):299‐305. - PubMed
ROTA council 2018
-
- ROTA council. Global Introduction Status. rotacouncil.org/vaccine‐introduction/global‐introduction‐status/ (accessed 27 July 2018).
Ruuska 1990
-
- Ruuska T, Vesikari T. Rotavirus disease in Finnish children: use of numerical scores for severity of diarrheal episodes. Scandinavian Journal of Infectious Diseases 1990;22(3):259‐67. - PubMed
SAGE 2009
-
- SAGE. Meeting of the immunization Strategic Advisory Group of Experts, April 2009‐‐conclusions and recommendations. Weekly Epidemiological Record 2009;84(23):220‐36. - PubMed
SAGE 2012
-
- SAGE. Meeting of the immunization Strategic Advisory Group of Experts, April 2012 ‐ conclusions and recommendations. Weekly Epidemiological Record 2012;21(87):201‐16. - PubMed
Sanderson 2011
-
- Sanderson C, Clark A, Taylor D, Bolanos B. Global review of rotavirus morbidity and mortality data by age and region. www.who.int/immunization/sage/meetings/2012/april/Sanderson_et_al_SAGE_A... 2011 (accessed 25 October 2018).
Schünemann 2017
-
- Schünemann HJ, Oxman AD, Vist GE, Higgins JP, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017). Cochrane, 2017. Available from www.cochrane.training.org/handbook.
Shui 2012
-
- Shui IM, Baggs J, Patel M, Parashar UD, Rett M, Belongia EA, et al. Risk of intussusception following administration of a pentavalent rotavirus vaccine in US infants. JAMA 2012;307(6):598‐604. - PubMed
Simonsen 2005
-
- Simonsen L, Viboud C, Elixhauser A, Taylor RJ, Kapikian AZ. More on RotaShield and intussusception: the role of age at the time of vaccination. Journal of Infectious Diseases 2005;192(Suppl 1):36‐43. - PubMed
Stowe 2016
-
- Stowe J, Andrews N, Ladhani S, Miller E. The risk of intussusception following monovalent rotavirus vaccination in England: A self‐controlled case‐series evaluation. Vaccine 2016;34(32):3684‐9. - PubMed
Tate 2012
-
- Tate JE, Burton AH, Boschi‐Pinto C, Steele AD, Duque J, Parashar UD, WHO‐coordinated Global Rotavirus Surveillance Network. 2008 estimate of worldwide rotavirus‐associated mortality in children younger than 5 years before the introduction of universal rotavirus vaccination programmes: a systematic review and meta‐analysis. Lancet Infectious Diseases 2012;12(2):136‐41. - PubMed
Tate 2018
Velázquez 1996
-
- Velázquez FR, Matson DO, Calva JJ, Guerrero L, Morrow AL, Carter‐Campbell S, et al. Rotavirus infection in infants as protection against subsequent infections. New England Journal of Medicine 1996;335(14):1022‐8. - PubMed
Velázquez 2017
Vesikari 1997
-
- Vesikari T. Rotavirus vaccines against diarrhoeal disease. Lancet 1997;350(9090):1538‐41. - PubMed
Vesikari 2015
-
- Vesikari T, Damme P, Giaquinto C, Dagan R, Guarino A, Szajewska H, et al. European Society for Paediatric Infectious Diseases consensus recommendations for rotavirus vaccination in Europe. Pediatric Infectious Disease Journal 2015;34(6):635‐43. - PubMed
Weintraub 2014
-
- Weintraub ES, Baggs J, Duffy J, Vellozzi C, Belongia EA, Irving S, et al. Risk of intussusception after monovalent rotavirus vaccination. New England Journal of Medicine 2014;370(6):513‐9. - PubMed
WHO 1999
-
- World Health Organization. List of Member States by WHO region and mortality stratum. www.who.int/whr/2003/en/member_states_182‐184_en.pdf (accessed on 04 April 2012).
WHO 2013
-
- World Health Organization. Rotavirus vaccines WHO position paper – January 2013. Weekly Epidemiological Record 2013;88(5):49‐64.
WHO 2018
-
- World Health Organization. WHO Prequalified Vaccines. extranet.who.int/gavi/PQ_Web/ (accessed 2 July 2018).
Yen 2014
Yen 2016
-
- Yen C, Healy K, Tate JE, Parashar UD, Bines J, Neuzil K, et al. Rotavirus vaccination and intussusception – Science, surveillance, and safety: a review of evidence and recommendations for future research priorities in low and middle income countries. Human Vaccines and Immunotherapeutics 2016;12(10):2580‐2589. - PMC - PubMed
Yih 2014
-
- Yih WK, Lieu TA, Kulldorff M, Martin D, McMahill‐Walraven CN, Platt R, et al. Intussusception risk after rotavirus vaccination in U.S. infants. New England Journal of Medicine 2014;370(6):503‐12. - PubMed
References to other published versions of this review
Soares‐Weiser 2004
Soares‐Weiser 2010
Soares‐Weiser 2012a
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

