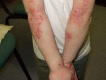
Interventions to reduce Staphylococcus aureus in the management of eczema
- PMID: 31684694
- PMCID: PMC6818407
- DOI: 10.1002/14651858.CD003871.pub3
Interventions to reduce Staphylococcus aureus in the management of eczema
Abstract
Background: Staphylococcus aureus (S. aureus) can cause secondary infection in eczema, and may promote inflammation in eczema that does not look infected. There is no standard intervention to reduce S. aureus burden in eczema. It is unclear whether antimicrobial treatments help eczema or promote bacterial resistance. This is an update of a 2008 Cochrane Review.
Objectives: To assess the effects of interventions to reduce S. aureus for treating eczema.
Search methods: We updated our searches of the following databases to October 2018: Cochrane Skin Group Specialised Register, CENTRAL, MEDLINE, Embase and LILACS. We searched five trials registers and three sets of conference proceedings. We checked references of trials and reviews for further relevant studies. We contacted pharmaceutical companies regarding ongoing and unpublished trials.
Selection criteria: Randomised controlled trials of products intended to reduce S. aureus on the skin in people diagnosed with atopic eczema by a medical practitioner. Eligible comparators were a similar treatment regimen without the anti-staphylococcal agent.
Data collection and analysis: We used standard methodological procedures expected by Cochrane. Our key outcomes were participant- or assessor-rated global improvement in symptoms/signs, quality of life (QOL), severe adverse events requiring withdrawal, minor adverse events, and emergence of antibiotic-resistant micro-organisms.
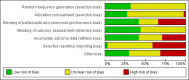
Main results: We included 41 studies (1753 analysed participants) covering 10 treatment categories. Studies were conducted mainly in secondary care in Western Europe; North America; the Far East; and elsewhere. Twelve studies recruited children; four, adults; 19, both; and six, unclear. Fifty-nine per cent of the studies reported the mean age of participants (range: 1.1 to 34.6 years). Eczema severity ranged from mild to severe. Many studies did not report our primary outcomes. Treatment durations ranged from 10 minutes to 3 months; total study durations ranged from 15 weeks to 27 months. We considered 33 studies at high risk of bias in at least one domain. We present results for three key comparisons. All time point measurements were taken from baseline. We classed outcomes as short-term when treatment duration was less than four weeks, and long-term when treatment was given for more than four weeks. Fourteen studies evaluated topical steroid/antibiotic combinations compared to topical steroids alone (infective status: infected (two studies), not infected (four studies), unspecified (eight studies)). Topical steroid/antibiotic combinations may lead to slightly greater global improvement in good or excellent signs/symptoms than topical steroid alone at 6 to 28 days follow-up (risk ratio (RR) 1.10, 95% confidence interval (CI) 1.00 to 1.21; 224 participants; 3 studies, low-quality evidence). There is probably little or no difference between groups for QOL in children, at 14 days follow-up (mean difference (MD) -0.18, 95% CI -0.40 to 0.04; 42 participants; 1 study, moderate-quality evidence). The subsequent results for this comparison were based on very low-quality evidence, meaning we are uncertain of their validity: severe adverse events were rare (follow-up: between 6 to 28 days): both groups reported flare of dermatitis, worsening of the condition, and folliculitis (325 participants; 4 studies). There were fewer minor adverse events (e.g. flare, stinging, itch, folliculitis) in the combination group at 14 days follow-up (218 participants; 2 studies). One study reported antibiotic resistance in children at three months follow-up, with similar results between the groups (65 participants; 1 study). Four studies evaluated oral antibiotics compared to placebo (infective status: infected eczema (two studies), uninfected (one study), one study's participants had colonisation but no clinical infection). Oral antibiotics may make no difference in terms of good or excellent global improvement in infants and children at 14 to 28 days follow-up compared to placebo (RR 0.80; 95% CI 0.18 to 3.50; 75 participants; 2 studies, low-quality evidence). There is probably little or no difference between groups for QOL (in infants and children) at 14 days follow-up (MD 0.11, 95% CI -0.10 to 0.32, 45 participants, 1 study, moderate-quality evidence). The subsequent results for this comparison were based on very low-quality evidence, meaning we are uncertain of their validity: adverse events requiring treatment withdrawal between 14 to 28 days follow-up were very rare, but included eczema worsening (both groups), loose stools (antibiotic group), and Henoch-Schönlein purpura (placebo group) (4 studies, 199 participants). Minor adverse events, including nausea, vomiting, diarrhoea, and stomach and joint pains, at 28 days follow-up were also rare and generally low in both groups (1 study, 68 infants and children). Antibiotic resistance at 14 days was reported as similar in both groups (2 studies, 98 infants and children). Of five studies evaluating bleach baths compared to placebo (water) or bath emollient (infective status: uninfected (two studies), unspecified (three studies)), one reported global improvement and showed that bleach baths may make no difference when compared with placebo at one month follow-up (RR 0.78, 95% CI 0.37 to 1.63; 36 participants; low-quality evidence). One study showed there is probably little or no difference in QOL at 28 days follow-up when comparing bleach baths to placebo (MD 0.90, 95% CI -1.32 to 3.12) (80 infants and children; moderate-quality evidence). We are uncertain if the groups differ in the likelihood of treatment withdrawals due to adverse events at two months follow-up (only one dropout reported due to worsening itch (placebo group)) as the quality of evidence was very low (1 study, 42 participants). One study reported that five participants in each group experienced burning/stinging or dry skin at two months follow-up, so there may be no difference in minor adverse events between groups (RR 1.00, 95% CI 0.35 to 2.87, 36 participants, low-quality evidence). Very low-quality evidence means we are also uncertain if antibiotic resistance at four weeks follow-up is different between groups (1 study, 80 participants ≤ 18 years).
Authors' conclusions: We found insufficient evidence on the effects of anti-staphylococcal treatments for treating people with infected or uninfected eczema. Low-quality evidence, due to risk of bias, imprecise effect estimates and heterogeneity, made pooling of results difficult. Topical steroid/antibiotic combinations may be associated with possible small improvements in good or excellent signs/symptoms compared with topical steroid alone. High-quality trials evaluating efficacy, QOL, and antibiotic resistance are required.
Copyright © 2019 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
Susannah MC George: none known. Sanja Karanovic: none known. David A Harrison: none known. Anjna Rani: none known. Andrew J Birnie has received payment from Leo Pharma and Almirall for delivering lectures on skin cancer and sun damage. Almirall has covered meeting registration fee, plus travel and accommodation, for the American Academy of Dermatology meeting in March 2017. Fiona J Bath‐Hextall: none known. Jane C Ravenscroft: none known. Hywel C Williams: I am director of the NIHR HTA Programme. HTA is part of the NIHR which also supports the NIHR systematic reviews programme from which this work is funded. I was also a co‐applicant on a relevant clinical trial (SCIN trial) that is not yet published that sought to prevent hand eczema in nurses, funded by the National Institute of Health Research Health Technology Assessment Programme
Figures

Update of
-
Interventions to reduce Staphylococcus aureus in the management of atopic eczema.Cochrane Database Syst Rev. 2008 Jul 16;(3):CD003871. doi: 10.1002/14651858.CD003871.pub2. Cochrane Database Syst Rev. 2008. Update in: Cochrane Database Syst Rev. 2019 Oct 29;2019(10). doi: 10.1002/14651858.CD003871.pub3. PMID: 18646096 Updated.
References
References to studies included in this review
Berman 2018 {published data only}
-
- Berman B, Nestor M. An investigator blinded randomized study evaluating hypochlorous acid (HOCl) in the treatment of atopic dermatitis‐associated pruritus. Journal of Clinical and Aesthetic Dermatology 2018;11(5 Supplement 1):S13.
Boguniewicz 2001 {published data only}
-
- Boguniewicz M, Sampson H, Leung SB, Harbeck R, Leung DYM. Effects of cefuroxime axetil on Staphylococcus aureus colonization and superantigen production in atopic dermatitis. Journal of Allergy and Clinical Immunology 2001;108(4):651‐2. [CENTRAL: CN‐00374302] - PubMed
Breneman 2000 {published data only}
-
- Breneman DL, Hanifin JM, Berge CA, Keswick BH, Neumann PB. The effect of antibacterial soap with 1.5% triclocarban on Staphylococcus aureus in patients with atopic dermatitis. Cutis 2000;66(4):296‐300. [CENTRAL: CN‐00372699] - PubMed
Canpolat 2012 {published data only (unpublished sought but not used)}
-
- Canpolat F, Erkocoglu M, Tezer H, Kocabas CN, Kandi B. Hydrocortisone acetate alone or combined with mupirocin for atopic dermatitis in infants under two years of age ‐ a randomized double blind pilot trial. European Review for Medical and Pharmacological Sciences 2012;16(14):1989‐93. [CENTRAL: CN‐00841489; PUBMED: 23242727] - PubMed
Daeschlein 2010 {published and unpublished data}
-
- Daeschlein G, Assadian O, Arnold A, Haase H, Kramer A, Junger M. Bacterial burden of worn therapeutic silver textiles for neurodermitis patients and evaluation of efficacy of washing. Skin Pharmacology and Physiology 2010;23(2):86‐90. [CENTRAL: CN‐00731215; PUBMED: 20016250] - PubMed
Ewing 1998 {published data only}
-
- Ewing CI, Ashcroft C, Gibbs ACC, Jones GA, Connor PJ, David TJ. Flucloxacillin in the treatment of atopic dermatitis. British Journal of Dermatology 1998;138(6):1022‐9. [CENTRAL: CN‐00155038] - PubMed
Fattah 1976 {published data only}
-
- Fattah AA, Shiemy S, Faris R. A comparative clinical evaluation of a new topical steroid "Halcinonide" and hydrocortisone in steroid responsive dermatoses. Journal of International Medical Research 1976;4(4):228‐31. [CENTRAL: CN‐00697652] - PubMed
Fluhr 2009 {published data only (unpublished sought but not used)}
-
- Fluhr JW, Breternitz M, Kowatzki D, Bauer A, Bossert J, Elsner P, et al. Silver‐loaded seaweed‐based cellulosic fiber improves epidermal skin physiology in atopic dermatitis: safety assessment, mode of action and controlled, randomized single‐blinded exploratory in vivo study. Experimental Dermatology 2010;19(8):e9‐15. [CENTRAL: CN‐00768275; PUBMED: 19645851] - PubMed
Foelster Holst 2010 {published data only}
-
- Foelster Holst R, Reitamo S, Yankova R, Worm M, Kadurina M, Thaci D, et al. The novel protease inhibitor SRD441 ointment is not effective in the treatment of adult subjects with atopic dermatitis: results of a randomized, vehicle‐controlled study. Allergy 2010;65(12):1594‐9. [CENTRAL: CN‐00772211; PUBMED: 21039597] - PubMed
Francis 2016 {published data only}
-
- Francis NA, Ridd MJ, Thomas‐Jones E, Shepherd V, Butler CC, Hood K, et al. A randomised placebo‐controlled trial of oral and topical antibiotics for children with clinically infected eczema in the community: the ChildRen with Eczema, Antibiotic Management (CREAM) study. Health Technology Assessment 2016;20(19):i‐xxiv, 1‐84. [CENTRAL: CN‐01259966; PUBMED: 26938214] - PMC - PubMed
Gauger 2006 {published data only}
-
- Gauger A, Fischer S, Mempel M, Schaefer T, Foelster‐Holst R, Abeck D, et al. Efficacy and functionality of silver coated textiles in patients with atopic eczema. Journal of the European Academy of Dermatology and Venereology 2006;20(5):534‐41. [CENTRAL: CN‐00565215] - PubMed
Gong 2006 {published data only}
-
- Gong JQ, Lin L, Lin T, Hao F, Zeng FQ, Bi ZG, et al. Skin colonization by Staphylococcus aureus in patients with eczema and atopic dermatitis and relevant combined topical therapy: a double‐blind multicentre randomized controlled trial. British Journal of Dermatology 2006;155(4):680‐7. [CENTRAL: CN‐00572111] - PubMed
Gonzalez 2016 {published and unpublished data}
-
- Gonzalez ME, Schaffer JV, Orlow SJ, Gao Z, Li H, Alekseyenko AV, et al. Cutaneous microbiome effects of fluticasone propionate cream and adjunctive bleach baths in childhood atopic dermatitis. Journal of the American Academy of Dermatology 2016;75(3):481‐493.e8. [CENTRAL: CN‐01368690; PUBMED: 27543211] - PMC - PubMed
Harper 1995 {published data only}
-
- Harper J. Double‐blind comparison of an antiseptic oil‐based bath additive (Oilatum Plus) with regular Oilatum (Oilatum Emollient) for the treatment of atopic eczema. In: Lever R, Levy J editor(s). The Bacteriology of Eczema: Round Table Series. Vol. 37, The Royal Society of Medicine Press Ltd, 1995:42‐47. [CENTRAL: CN‐00184126]
Hizawa 1998 {published data only}
-
- Hizawa T, Sano H, Endo K, Fukuzumi T, Kataoka Y, Aoki T. Is povidone‐iodine effective to the lesions of atopic dermatitis. Hifu [Skin Research] 1998;40(Suppl 20):134‐9. [CENTRAL: CN‐00499393]
Hjorth 1985 {published data only}
-
- Hjorth N, Schmidt H, Thomsen K. Fusidic acid plus betamethasone in infected or potentially infected eczema. Pharmacotheraputica 1985;4(2):126‐31. [CENTRAL: CN‐00259287] - PubMed
Holland 1995 {published data only}
-
- Holland KT, Bojar RA, Cunliffe WJ. A comparison of the effect of treatment of atopic eczema with and without antimicrobial compounds. In: Lever R, Levy J editor(s). The Bacteriology of Eczema: Round Table Series. Vol. 37, The Royal Society of Medicine Press Ltd, 1995:37‐40. [CENTRAL: CN‐00747631]
Hon 2016 {published data only}
-
- Hon KL, Tsang YC, Lee VW, Pong NH, Ha G, Lee ST, et al. Efficacy of sodium hypochlorite (bleach) baths to reduce Staphylococcus aureus colonization in childhood onset moderate‐to‐severe eczema: a randomized, placebo‐controlled cross‐over trial. Journal of Dermatological Treatment 2016;27(2):156‐62. [CENTRAL: CN‐01132944; PUBMED: 26270469] - PubMed
Huang 2009 {published data only}
-
- Huang JT, Abrams M, Tlougan B, Rademaker A, Paller AS. Treatment of Staphylococcus aureus colonization in atopic dermatitis decreases disease severity. Pediatrics 2009;123(5):e808‐14. [CENTRAL: CN‐00700251; PUBMED: 19403473] - PubMed
-
- Huang JT, Rademaker A, Paller AS. Dilute bleach baths for Staphylococcus aureus colonization in atopic dermatitis to decrease disease severity. Archives of Dermatology 2011; Vol. 147, issue 2:246‐7. [PUBMED: 21339459] - PubMed
Hung 2007 {published data only}
-
- Hung SH, Lin YT, Chu CY, Lee CC, Liang TC, Yang YH, et al. Staphylococcus colonization in atopic dermatitis treated with fluticasone or tacrolimus with or without antibiotics. Annals of Allergy, Asthma & Immunology 2007;98(1):51‐6. [CENTRAL: CN‐00574988; MEDLINE: ] - PubMed
Juenger 2006 {published data only}
-
- Juenger M, Ladwig A, Staecker S, Arnold A, Kramer A, Daeschlein G, et al. Efficacy and safety of silver textile in the treatment of atopic dermatitis (AD). Current Medical Research and Opinion 2006;22(4):739‐50. [CENTRAL: CN‐00565217] - PubMed
Koller 2007 {published data only (unpublished sought but not used)}
-
- Koller DY, Halmerbauer G, Bock A, Engstler G. Action of a silk fabric treated with AEGIS in children with atopic dermatitis: a 3‐month trial. Pediatric Allergy and Immunology 2007;18(4):335‐8. [CENTRAL: CN‐00636754; PUBMED: 17346297] - PubMed
Korting 1994 {published data only}
-
- Korting HC, Zienicke H, Braun‐Falco O, Boek K, Milbradt R, Nolting S, et al. Modern topical glucocorticoids and anti‐infectives for superinfected atopic eczema: do prednicarbate and didecyldimethylammoniumchloride form a rational combination?. Infection 1994;22(6):390‐3. [CENTRAL: CN‐00499406] - PubMed
-
- Zienicke H. Topical glucocorticoids and anti‐infectives: a rational combination?. Current Problems in Dermatology 1993;21:186‐91. [CENTRAL: CN‐00098710] - PubMed
Leins 2013 {published and unpublished data}
-
- Leins E, Scullin M. Bleach baths for eczema. Australasian Journal of Dermatology 2013;54(Suppl 2):55.
Lembo 2011 {published and unpublished data}
-
- Lembo C, Patruno C, Leonibus C, Panariello L, Lembo S, Ayala F. Topical erythromycin in atopic dermatitis [L'eritromicina topica nella terapia della dermatite atopica]. Annali Italiani di Dermatologia Allergologica Clinica e Sperimentale 2011;65(3):113‐8. [CENTRAL: CN‐00843852]
Lever 1988 {published data only}
-
- Lever R, Hadley K, Downey D, Mackie R. Staphylococcal colonization in atopic dermatitis and the effect of topical mupirocin therapy. British Journal of Dermatology 1988;119(2):189‐98. [CENTRAL: CN‐00055801] - PubMed
Leyden 1977 {published data only}
-
- Leyden JJ, Kligman AM. The case for steroid‐antibiotic combinations. British Journal of Dermatology 1977;96(2):179‐87. [CENTRAL: CN‐00569045] - PubMed
Lopes 2015 {published data only}
Masako 2005b {published data only}
-
- Masako K, Yusuke K, Hideyuki I, Atsuko M, Yoshiki M, Kayoko M, et al. A novel method to control the balance of skin microflora. Part 2. A study to assess the effect of a cream containing farnesol and xylitol on atopic dry skin. Journal of Dermatological Science 2005;38(3):207‐13. [CENTRAL: CN‐00740969] - PubMed
Nilsson 1992 {published data only}
-
- Nilsson EJ, Henning CG, Magnusson J, Sundsvall BS. Topical corticosteroids and Staphylococcus aureus in atopic dermatitis. Journal of the American Academy of Dermatology 1992;27(1):29‐34. [CENTRAL: CN‐00360692] - PubMed
Polano 1960 {published data only}
-
- Polano MK, Vries HR, Delver A. Analysis of the results obtained in the treatment of atopic dermatitis with corticosteroid and neomycin containing ointments. Dermatologica 1960;120:191‐9. [CENTRAL: CN‐00360818] - PubMed
Portela Araujo 2013 {published data only (unpublished sought but not used)}
-
- Araujo CP, Gomes J, Vieira AP, Ventura F, Fernandes JC, Brito C. A proposal for the use of new silver‐seaweed‐cotton fibers in the treatment of atopic dermatitis. Cutaneous and Ocular Toxicology 2013;32(4):268‐74. [CENTRAL: CN‐00918329; PUBMED: 23485342] - PubMed
Ramsay 1996 {published data only}
-
- Ramsay CA, Savoie JM, Gilbert M, Gidon M, Kidson P. The treatment of atopic dermatitis with topical fusidic acid and hydrocortisone acetate. Journal of the European Academy of Dermatology and Venereology 1996;7(Suppl 1):S15‐22. [CENTRAL: CN‐00172818]
Schempp 2003 {published data only}
-
- Schempp CM, Hezel S, Simon JC. Topical treatment of atopic dermatitis with St John's wort cream ‐ a randomized, placebo controlled, double blind half‐side comparison [Behandlung der subakuten atopischen Dermatitis mit Johanniskraut‐Creme. Eine randomisierte, placebokontrollierte Doppelblindstudie im Halbsetendesign]. Hautarzt 2003;54(3):248‐53. [CENTRAL: CN‐00509733] - PubMed
-
- Schempp CM, Windeck T, Hezel S, Simon JC. Topical treatment of atopic dermatitis with St John's wort cream ‐ a randomized, placebo controlled, double blind half‐side comparison. Phytomedicine 2003;10(Suppl IV):31‐7. [CENTRAL: CN‐00438398] - PubMed
Schuttelaar 2005 {published and unpublished data}
-
- Schuttelaar ML, Coenraads P‐J. A randomised, double‐blind study to assess the efficacy of addition of tetracycline to triamcinolone acetonide (class II corticosteroid) in the treatment of moderate to severe atopic dermatitis. Journal of the European Academy of Dermatology and Venereology 2008;22(9):1076‐82. [CENTRAL: CN‐00667932] - PubMed
Shi 2016 {published data only}
-
- Shi VY, Foolad N, Ornelas JN, Hassoun LA, Monico G, Takeda N, et al. Comparing the effect of bleach and water baths on skin barrier function in atopic dermatitis: a split‐body randomized controlled trial. British Journal of Dermatology 2016;175(1):212‐4. [CENTRAL: CN‐01383031; PUBMED: 26875771] - PubMed
Stinco 2008 {published data only}
-
- Stinco G, Piccirillo F, Valent F. A randomized double‐blind study to investigate the clinical efficacy of adding a non‐migrating antimicrobial to a special silk fabric in the treatment of atopic dermatitis. Dermatology 2008;217(3):191‐5. [CENTRAL: CN‐00668416; PUBMED: 18583910] - PubMed
Tan 2009 {published data only}
-
- Tan WP, Suresh S, Tey HL, Chiam LY, Goon AT. A randomized double‐blind controlled trial to compare a triclosan‐containing emollient with vehicle for the treatment of atopic dermatitis. Clinical and Experimental Dermatology 2010;35(4):e109‐12. [CENTRAL: CN‐00762014; PUBMED: 19843084] - PubMed
Wachs 1976 {published data only}
-
- Wachs GN, Maibach HI. Co‐operative double‐blind trial of an antibiotic/corticosteroid combination in impetiginized atopic dermatitis. British Journal of Dermatology 1976;95(3):323‐8. [CENTRAL: CN‐00014807] - PubMed
Weinberg 1992 {published data only}
-
- Weinberg E, Fourie B, Allmann B, Toerien A. The use of cefadroxil in superinfected atopic dermatitis. Current Therapeutic Research, Clinical and Experimental 1992;52(5):671‐6. [CENTRAL: CN‐00195530]
Wong 2013 {published and unpublished data}
-
- Wong SM, Ng TG, Baba R. Efficacy and safety of sodium hypochlorite (bleach) baths in patients with moderate to severe atopic dermatitis in Malaysia. Journal of Dermatology 2013;40(11):874‐80. [CENTRAL: CN‐00915612; PUBMED: 24111816] - PubMed
References to studies excluded from this review
Alangari 2017 {published data only}
Ariyoshi 1973 {published data only}
-
- Ariyoshi M, Matsumoto T. A clinical trial of amoxycillin in some infectious skin diseases. Chemotherapy 1973;21(8):1812‐6.
Berardesca 2009 {published data only}
-
- Berardesca E, Abril E, Serio M, Cameli N. Effects of topical gluco‐oligosaccharide and collagen tripeptide F in the treatment of sensitive atopic skin. International Journal of Cosmetic Science 2009;31(4):271‐7. [PUBMED: 19496838] - PubMed
Bergstrom 2009 {published data only}
-
- Bergstrom KG. Tea tree oil: panacea or placebo?. Journal of Drugs in Dermatology 2009;8(5):494‐6. [PUBMED: 19537376] - PubMed
Bianchi 2014 {published data only}
-
- Bianchi P, Villeneuve C, Theunis J, Casas C, Patrizi A, Bacquey A, et al. Evaluation of a novel emollient balm containing Aquaphilus dolomiae extract in atopic dermatitis children: effect on clinical parameters, skin barrier and microflora. Journal of Investigative Dermatology 2014;134(Suppl 1):S96.
Bjornberg 1975 {published data only}
-
- Bjornberg A, Hellgren L, Nygren B. Diproderm with Gentamicin ‐ a new very potent steroid ointment in infected eczema. Current Therapeutic Research 1975;18(4):556‐8. [PUBMED: 810315] - PubMed
Breneman 1990 {published data only}
-
- Breneman DL. Use of mupiricin ointment in the treatment of secondarily infected dermatoses. Journal of the American Academy of Dermatology 1990;22(5 Pt 1):886‐92. [PUBMED: 2112167] - PubMed
Carpenter 1973 {published data only}
-
- Carpenter CL, Jolly HW, McCormick GE, Schneiderman RN, Thompson JG. Combined steroid‐antiinfective topical therapy in common dermatoses: a double‐blind, multicenter study of iodochlorhydroxyquin‐hydrocortisone in 277 patients. Current Therapeutic Research 1973;15(9):650‐9. [PUBMED: 4270829] - PubMed
Clark 1974 {published data only}
-
- Clark RF. The case for corticosteroid‐antibiotic combinations. Cutis 1974;14(5):737‐41. [CENTRAL: CN‐00269373]
Craig 2010 {published data only}
-
- Craig FE, Smith EV, Williams HC. Bleach baths to reduce severity of atopic dermatitis colonized by Staphylococcus. Archives of Dermatology 2010;146(5):541‐3. [PUBMED: 20479303] - PubMed
Davis 1968 {published data only}
-
- Davis CM, Fulghum DD, Taplin D. The value of neomycin in a neomycin‐steroid cream. JAMA 1968;203(4):298‐300. [CENTRAL: CN‐00651515] - PubMed
Drago 2011 {published data only}
-
- Drago L, Iemoli E, Rodighiero V, Nicola L, Vecchi E, Piconi S. Effects of Lactobacillus salivarius LS01 (DSM 22775) treatment on adult atopic dermatitis: a randomized placebo‐controlled study. International Journal of Immunopathology and Pharmacology 2011;24(4):1037‐48. [PUBMED: 22230409] - PubMed
Drago 2012 {published data only}
-
- Drago L, Toscano M, Vecchi E, Piconi S, Iemoli E. Changing of fecal flora and clinical effect of L. salivarius LS01 in adults with atopic dermatitis. Journal of Clinical Gastroenterology 2012;46 Suppl:S56‐63. [CENTRAL: CN‐00967749; PUBMED: 22955359] - PubMed
Dunstan 2011 {published data only}
Eaglstein 1977 {published data only}
-
- Eaglstein WH, Feinstein RJ, Halprin KM, Bergstresser PR, Mertz PM. Systemic antibiotic therapy of secondary infected dermatitis. Archives of Dermatology 1977;113(10):1378‐9. [CENTRAL: CN‐00016805] - PubMed
Gauger 2003 {published data only}
-
- Gauger A, Mempel M, Schekatz A, Schafer T, Ring J, Abeck D. Silver‐coated textiles reduce Staphylococcus aureus colonization in patients with atopic eczema. Dermatology 2003;207(1):15‐21. [CENTRAL: CN‐00438876] - PubMed
Girolomoni 2016 {published data only}
-
- Girolomoni G, Mattina R, Manfredini S, Vertuani S, Fabrizi G. Fusidic acid betamethasone lipid cream. International Journal of Clinical Practice 2016;70(Suppl 184):4‐13. [PUBMED: 27121235] - PubMed
GP Medical Research 1967 {published data only}
-
- GP Medical Research Unit. Treatment of eczemas and infected eczemas. British Journal of Clinical Practice 1967;21(10):505‐7. [CENTRAL: CN‐00001629] - PubMed
Gratton 1987 {published data only}
-
- Gratton D. Topical mupirocin versus oral erythromycin in the treatment of primary and secondary skin infections. International Journal of Dermatology 1987;26(7):472‐3. [CENTRAL: CN‐00268360; PUBMED: 3115904] - PubMed
Gueniche 2008 {published data only}
-
- Gueniche A, Knaudt B, Schuck E, Volz T, Bastien P, Martin R, et al. Effects of nonpathogenic gram‐negative bacterium Vitreoscilla filiformis lysate on atopic dermatitis: a prospective, randomized, double‐blind, placebo‐controlled clinical study. British Journal of Dermatology 2008;159(6):1357‐63. [CENTRAL: CN‐00665626; PUBMED: 18795916] - PubMed
Gueniche 2009 {published data only}
-
- Gueniche A, Bastien P, Mahe Y, Billoni N, Knaudt B, Piche E, et al. Antimicrobial activity of Vitreoscilla filiformis lysate: results from a randomized, double‐blind, vehicle‐controlled and in vitro study. Journal of Investigative Dermatology 2009;129(Suppl 2s):S18. [CENTRAL: CN‐00790672]
Hoey 2006 {published data only}
-
- Hoey SEH, Catney D, Maguire S, McKenna K. Fixed low dose versus increasing dose of ultraviolet B‐TL01 in the treatment of atopic eczema. British Journal of Dermatology 2006;155(Suppl 1):121‐2. [CENTRAL: CN‐00602220]
Ishibashi 1993 {published data only}
-
- Ishibashi Y, Harada S, Arata J, Niimura M, Takeda K, Shimao S, et al. Clinical utility of DS‐2630 ointment on oozing eczema‐dermatitis: a double‐blind bilateral‐paired comparison study with 0.064% betamethasone diproprionate ointment. Journal of Clinical Therapeutics and Medicines 1993;9(7):1583‐601. [CENTRAL: CN‐00547143]
Kimata 1998 {published data only}
-
- Kimata H. Effect of nadifloxacin on atopic dermatitis with methicillin‐resistant Staphylococcus aureus in young children. European Journal of Pediatrics 1999;158(11):949‐54. [CENTRAL: CN‐00263884] - PubMed
Kotrajaras 1971 {published data only}
-
- Kotrajaras R. Studies of bacterial infections of skin in Bangkok. International Journal of Dermatology 1973;12(3):163‐5. [CENTRAL: CN‐00568720; PUBMED: 4267413] - PubMed
La Colla 2009 {published data only}
-
- Colla L, Mangano A, Mangano A, Albertin A. Effects of nonpathogenic gram‐negative bacterium Vitreoscilla filiformis lysate on atopic dermatitis: a prospective, randomized, double‐blind, placebo‐controlled clinical study. Does this make a real difference?. British Journal of Dermatology 2009;161(2):477‐9. [PUBMED: 19485997] - PubMed
Leung 2008 {published data only}
-
- Leung TF, Wong KY, Wong CK, Fung KP, Lam CW, Fok TF, et al. In vitro and clinical immunomodulatory effects of a novel Pentaherbs concoction for atopic dermatitis. British Journal of Dermatology 2008;158(6):1216‐23. [PUBMED: 18341655] - PubMed
Leung 2009 {published data only}
-
- Leung DY, Hanifin JM, Pariser DM, Barber KA, Langley RG, Schlievert PM, et al. Effects of pimecrolimus cream 1% in the treatment of patients with atopic dermatitis who demonstrate a clinical insensitivity to topical corticosteroids: a randomized, multicentre vehicle‐controlled trial. British Journal of Dermatology 2009;161(2):435‐43. [CENTRAL: CN‐00728093; PUBMED: 19416245] - PubMed
Lloyd 1969 {published data only}
-
- Lloyd KM. The value of neomycin in topical corticosteroid preparations. Southern Medical Journal 1969;62(1):94‐6. [CENTRAL: CN‐00307468] - PubMed
Masako 2005a {published data only}
-
- Katsuyama M, Ichikawa H, Ogawa S, Ikezawa Z. A novel method to control the balance of skin microflora. Part 1. Attack on biofilm of Staphylococcus aureus without antbiotics. Journal of Dermatological Science 2005;38(3):197‐205. [PUBMED: 15927813] - PubMed
Meenan 1988 {published data only}
-
- Meenan FO. A double‐blind comparative study to compare the efficacy of Locoid C with Tri‐adcortyl in chldren with infected eczema. British Journal of Clinical Practice 1988;42(5):200‐2. [PUBMED: 2463849] - PubMed
Mora 2004 {published data only}
-
- Mora R, Bellussi L, Passali FM, Crippa B, Mora F, Cordone MP, et al. Efficacy of a topical suspension of bacterial antigens for the management of recurrent eczema in children. Medical Science Monitor 2004;10(9):PI99‐103. [CENTRAL: CN‐00501644; PUBMED: 15328495] - PubMed
Nielsen 1979 {published data only}
-
- Nielsen R, Thomsen K. Corticosteroid preparations with and without supplementary antibiotics. Comparison of fluclorolone acetonide (Topilar cream) and betamethasone valerate cream (Betnovat) with neomycin [Kortikosteroidpraeparater med og uden antibiotikumtilsaetning. En sammenligning mellem fluklorolon acetonid (Topilar creme) og cremor betametasonvalerat (Betnovate) med neomycin]. Ugeskrift For Laeger 1979;141(30):2046‐9. [CENTRAL: CN‐00022116; PUBMED: 531939] - PubMed
Parish 1987 {published data only}
-
- Parish LC, Parks DB, Layne PP, Uri JV. Ceftizoxime treatment of cutaneous and subcutaneous tissue infections. Clinical Therapeutics 1984;6(5):613‐9. [CENTRAL: CN‐00177354; PUBMED: 6090019] - PubMed
Pratap 2013 {published and unpublished data}
-
- Pratap DV, Philip M, Rao NT, Jerajani HR, Kumar SA, Kuruvila M, et al. Evaluation of efficacy, safety, and tolerability of fixed dose combination (FDC) of Halometasone 0.05% and Fusidic Acid 2% W/W topical cream versus FDC of Betamethasone Valerate 0.12% and Neomycin Sulphate 0.5% W/W topical cream in the treatment of infected eczematous dermatosis in Indian subjects: a randomized open‐label comparative phase III multi‐centric trial. Indian Journal of Dermatology 2013;58(2):117‐23. [CENTRAL: CN‐01092632; PUBMED: 23716800] - PMC - PubMed
Prescott 2005 {published data only}
-
- Prescott SL, Dunstan JA, Hale J, Breckler L, Lehmann H, Weston S, et al. Clinical effects of probiotics are associated with increased interferon‐gamma responses in very young children with atopic dermatitis. Clinical and Experimental Allergy 2005;35(12):1557‐64. [PUBMED: 16393321] - PubMed
Ravenscroft 2003 {published data only}
-
- Ravenscroft JC, Layton AM, Eady EA, Murtagh MS, Coates P, Walker M, et al. Short‐term effects of topical fusidic acid or mupirocin on the prevalence of fusidic acid resistant (FusR) Staphylococcus aureus in atopic eczema. British Journal of Dermatology 2003;148(5):1010‐7. [CENTRAL: CN‐00558784; PUBMED: 12786834] - PubMed
Remitz 2001 {published data only}
-
- Remitz A, Kyllonen H, Granlund H, Reitamo S. Tacrolimus ointment reduces staphylococcal colonization of atopic dermatitis lesions. Journal of Allergy and Clinical Immunology 2001;107(1):196‐7. [PUBMED: 11150013] - PubMed
Rist 2002 {published data only}
-
- Rist T, Parish LC, Capin LR, Sulica V, Bushnell WD, Cupo MA. A comparison of the efficacy and safety of mupirocin cream and cepalexin in the treatment of secondarily infected eczema. Clinical and Experimental Dermatology 2002;27(1):14‐20. [PUBMED: 11952661] - PubMed
Salo 1988 {published data only}
-
- Salo OP, Gordin A, Brandt H, Antikainen R. Efficacy and tolerability of erythromycin acistrate and erythromycin stearate in acute skin infections of patients with atopic eczema. Journal of Antimicrobial Chemotherapy 1988;21(Suppl D):101‐6. [CENTRAL: CN‐00054664] - PubMed
Sandström Falk 2006 {published data only}
-
- Sandstrom Falk MH, Sarnhult T, Hedner T, Faergemann J. Treatment of atopic dermatitis with 1% hydrocortisone and 25% pentane‐1,5‐diol: effect on Staphylococcus aureus. Acta Dermato‐Venereologica 2006;86(4):372‐3. [PUBMED: 16874433] - PubMed
Sasai‐Takedatsu 1997 {published data only}
-
- Sasai‐Takedatsu M, Kojima T, Yamamoto A, Hattori K, Yoshijima S, Taniuchi S, et al. Reduction of Staphylococcus aureus in atopic skin lesions with acid electrolytic water ‐ a new therapeutic strategy for atopic dermatitis. Allergy 1997;52(10):1012‐6. [CENTRAL: CN‐00145059] - PubMed
Schempp 2010 {published data only}
-
- Schempp C, Wölfle U. Hyperforin ‐ a multi‐talent for the skin [Hyperforin ‐ ein multitalent für die haut]. Der Hautarzt; Zeitschrift fur Dermatologie, Venerologie, und Verwandte Gebiete 2010;21(4):178‐80.
Schultz Larsen 2007 {published data only}
-
- Larsen FS, Simonsen L, Melgaard A, Wendicke K, Henriksen AS. An efficient new formulation of fusidic acid and betamethasone 17‐valerate (fucicort lipid cream) for treatment of clinically infected atopic dermatitis. Acta Dermato‐Venereologica 2007;87(1):62‐8. [CENTRAL: CN‐00586553; PUBMED: 17225018] - PubMed
Senti 2006 {published data only}
-
- Senti G, Steinmann LS, Fischer B, Kurman R, Storni T, Johansen P, et al. Antimicrobial silk clothing in the treatment of atopic dermatitis proves comparable to topical corticosteroid treatment. Dermatology 2006;213(3):228‐33. [PUBMED: 17033173] - PubMed
Stalder 1992 {published data only}
-
- Stalder JF, Fleury M, Sourisse M, Allavoine TH, Chalamet C, Brosset P, et al. Comparative effects of two topical antiseptics (chlorhexidine vs KMnO4) on bacterial skin flora in atopic dermatitis. Acta Dermato‐Venereologica 1992;176(Suppl):132‐4. [CENTRAL: CN‐00089825] - PubMed
Takahama 1992 {published data only}
-
- Takahama H, Wagatsuma K, Takahashi H, YamIshiada N, Torii H, Ishibashi Y. Clinical trial of ME1207 in the dermatological field. Chemotherapy 1992;40(Suppl 2):805‐8.
Thaci 1999 {published data only}
-
- Thaci D, Kokorsch J, Kaufmann R. Fusidic acid/Betamethasone 17‐valerate in potentially infected atopic dermatitis. Journal of the European Academy of Dermatology 1999;12(Suppl 2):S163. [CENTRAL: CN‐00478779]
Theodoridis 1979 {published data only}
-
- Theodoridis A, Vagena A, Sivenas C, Capetanakis J. Evaluation of a topical steroid antibiotic combination (halcinonide‐neomycin‐amphotericin) in the treatment of cutaneous candidiasis and inflammatory dermatoses. Current Medical Research and Opinion 1979;5(10):766‐71. [CENTRAL: CN‐00020280] - PubMed
Thum 2013 {published data only}
-
- Thum D, Seidl HP, Hein R, Ring J, Andres C, Mempel M. Current resistance patterns of Staphylococcus aureus towards topical antibiotics and relevant antiseptics in patients with atopic dermatitis and impetigo. Journal der Deutschen Dermatologischen Gesellschaft 2013;11(9):875‐8. [PUBMED: 23621472] - PubMed
Udompataikul 2015 {published data only}
-
- Udompataikul M, Huajai S, Chalermchai T, Taweechotipatr M, Kamanamool N. The effects of oral vitamin D supplement on atopic dermatitis: a clinical trial with staphylococcus aureus colonization determination. Journal of the Medical Association of Thailand 2015;98(Suppl 9):S23‐30. [PUBMED: 26817206] - PubMed
Van der Bijl 1966 {published data only}
-
- Bijl WJ. A policlinical study of a new external corticosteroid preparation Locacorten. Acta Allergologica 1966;21(6):515‐21. [PUBMED: 6012554] - PubMed
Verallo‐Rowell 2008 {published data only}
-
- Verallo‐Rowell VM, Dillague KM, Syah‐Tjundawan BS. Novel antibacterial and emollient effects of coconut and virgin olive oils in adult atopic dermatitis. Dermatitis 2008;19(6):308‐15. [CENTRAL: CN‐00681044; PUBMED: 19134433] - PubMed
Weitgasser 1983 {published data only}
-
- Weitgasser H, Schindlery C, Macarol V. A comparative multicentre trial of halometasone/triclosan cream and betamethasone diproprionate/gentamicin sulphate cream in the treatment of infected acute eczematous dermatitis. Journal of International Medical Research 1983;11(Suppl 1):43‐7. [CENTRAL: 6339292] - PubMed
Whitefield 1998 {published data only}
-
- Whitefield M. Effectiveness of a new antimicrobial emollient in the management of eczema/dermatitis. Journal of Dermatological Treatment 1998;9(2):103‐9. [CENTRAL: CN‐00261088]
Wilkinson JD 1985 {published data only}
-
- Wilkinson JD, Leigh DA. Comparative efficacy of betamethasone and either fusidic acid or neomycin in infected or potentially infected eczema. Current Therapeutic Research 1985;38(1):177‐82. [CENTRAL: CN‐00350766]
Wilkinson RD 1980 {published data only}
-
- Wilkinson RD, Collins JP, Raymond GP, Mailhot R, Perras MA, Ricard P. Therapy of infected dermatitis: comparative response to two corticosteroid antimicrobial creams. Journal of the American Academy of Dermatology 1980;2(3):207‐11. [CENTRAL: CN‐00022458] - PubMed
References to studies awaiting assessment
ACTRN12610000438055 {published data only}
-
- ACTRN12610000438055. A randomised, double‐blind, placebo‐controlled phase I study to determine the safety and efficacy of recombinant human ß defensin 2 (rHuB[beta]D2 cream) in the treatment of staphylococcus aureus and other infections in the skin of patients with atopic dermatitis. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=335452 (first received 6 May 2010).
EudraCT 2006‐004233‐15 {published data only}
-
- 2006‐004233‐15. Multicentric, randomized, double‐blinded, vehicle‐controlled, phase III‐bilateral comparative study for treatment of staphylococcus superinfection in atopic dermatitis with hydrophobic Triclosan‐cream 2% (InfectoDermaSept). www.clinicaltrialsregister.eu/ctr‐search/search?query=2006‐004233‐15 (first received 16 April 2009).
EudraCT 2008‐005890‐37 {published data only}
-
- 2008‐005890‐37. Assessment of the effects on barrier impairment, clinical features and bacterial colonization of topical formulations in patients with atopic eczema; a phase IIa, single‐center, randomized, observer‐blinded study. www.clinicaltrialsregister.eu/ctr‐search/search?query=2008‐005890‐37 (first received 20 October 2008).
NCT03009734 {published data only}
-
- NCT03009734. Evaluation of ATx201 as a topical antibiotic agent. clinicaltrials.gov/ct2/show/NCT03009734 (first received 20 March 2018).
NCT03047954 {published data only}
-
- NCT03047954. Broncho‐Vaxom (OM 85 BV) in children suffering from atopic dermatitis. clinicaltrials.gov/ct2/show/NCT03047954 (first received 9 February 2017).
Totté 2017 {published data only}
-
- NCT02840955. The effect of gladskin on disease severity and the skin microbiome, including Staphylococcus aureus, in patients with atopic dermatitis. clinicaltrials.gov/ct2/show/NCT02840955 (first received 11 July 2016).
-
- Totté J, Wit J, Pardo L, Schuren F, Doorn M, Pasmans S. Targeted anti‐staphylococcal therapy with endolysins in atopic dermatitis and the effect on steroid use, disease severity and the microbiome: study protocol for a randomized controlled trial (MAAS trial). Trials 2017;18(1):404. [PUBMED: 28859690] - PMC - PubMed
References to ongoing studies
NCT03052348 {published data only}
-
- NCT03052348. Efficacy of combining topical antibiotic/steroid/moisturizer therapy compared to active comparator in atopic dermatitis. clinicaltrials.gov/ct2/show/NCT03052348 (first received 10 February 2017).
Additional references
Antonov 2015
Barbarot 2016
-
- Barbarot S, Rogers NK, Abuabara K, Aubert H, Chalmers J, Flohr C, et al. Strategies used for measuring long‐term control in atopic dermatitis trials: a systematic review. Journal of the American Academy of Dermatology 2016;75(5):1038‐44. [PUBMED: 27522613] - PubMed
Benetti 2015
BNF 2019
-
- Joint Formulary Committee. British National Formulary. www.bnf.org/products/bnf‐online/ (accessed prior to 23 October 2019).
Brown 2002
-
- Brown EM, Thomas P. Fusidic acid resistance in Staphylococcus aureus isolates. Lancet 2002;359(9308):803. [PUBMED: 11888633] - PubMed
Chalmers 2014
-
- Chalmers JR, Schmitt J, Apfelbacher C, Dohil M, Eichenfield LF, Simpson EL, et al. Report from the third international consensus meeting to harmonise core outcome measures for atopic eczema/dermatitis clinical trials (HOME). British Journal of Dermatology 2014;171(6):1318‐25. [PUBMED: 24980543] - PMC - PubMed
Chaptini 2015
Charman 2004
-
- Charman CR, Venn AJ, Williams HC. The patient‐oriented eczema measure: development and initial validation of a new tool for measuring atopic eczema severity from the patients' perspective. Archives of Dermatology 2004;140(12):1513‐9. [PUBMED: 15611432] - PubMed
Chopra 2017
Costa 1989
-
- Costa C, Rilliet A, Nicolet M, Saurat JH. Scoring atopic dermatitis: the simpler the better?. Acta Dermato‐Venereologica 1989;69(1):41‐5. [PUBMED: 2563607] - PubMed
David 1986
Deckers 2012
Eichenfield 2003
-
- Eichenfield LF, Hanifin JM, Luger TA, Stevens SR, Pride HB. Consensus conference on pediatric atopic dermatitis. Journal of the American Academy of Dermatology 2003;49(6):1088‐95. [PUBMED: 14639390] - PubMed
European Task Force on Atopic Dermatitis 1993
-
- European Task Force on Atopic Dermatitis. Severity scoring of atopic dermatitis: the SCORAD index. Consensus Report. Dermatology 1993;186(1):23‐31. [PUBMED: 8435513] - PubMed
Finlay 1994
-
- Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI) ‐ a simple practical measure for routine clinical use. Clinical and Experimental Allergy 1994;19(3):210‐6. [PUBMED: 8033378] - PubMed
George 2015
GRADE Working Group 2004
Hanifin 1980
-
- Hanifin JM, Rajka G. Diagnostic features of atopic eczema. Acta Dermato‐Venerologica 1980;92:44‐7.
Hanifin 2001
-
- Hanifin JM, Thurston M, Omoto M, Cherill R, Tofte SJ, Graeber M, EASI Evaluator Group. The eczema area and severity index (EASI): assessment of reliability in atopic dermatitis. Experimental Dermatology 2001;10(1):11‐8. [PUBMED: 11168575] - PubMed
Hann 2007
-
- Hann S, Hughes TM, Stone NM. Flexural allergic contact dermatitis to benzalkonium chloride in antiseptic bath oil. British Journal of Dermatology 2007;157(4):795‐8. [PUBMED: 17714561 ] - PubMed
Heng 2013
Hepburn 2017
-
- Hepburn L, Hijnen DJ, Sellman BR, Mustelin T, Sleeman MA, May RD, et al. The complex biology and contribution of Staphylococcus aureus in atopic dermatitis, current and future therapies. British Journal of Dermatology 2017;177(1):63‐71. [PUBMED: 27779765] - PubMed
Higgins 2011
-
- Higgins JPT, Green S, (editors). Cochrane Handbook for Systematic Reviews of Interventions. 5.1.0 [updated March 2011]. Chichester (UK): John Wiley & Sons, 2011.
Irvine 2006
-
- Irvine AD, McLean WH. Breaking the (un)sound barrier: filaggrin is a major gene for atopic dermatitis. Journal of Investigative Dermatology 2006;126(6):1200‐2. [PUBMED: 16702964] - PubMed
Jagadeesan 2014
-
- Jagadeesan S, Kurien G, Divakaran MV, Sadanandan SM, Sobhanakumari K, Sarin A. Methicillin‐resistant Staphylococcus aureus colonization and disease severity in atopic dermatitis: a cross‐sectional study from South India. Indian Journal of Dermatology, Venereology and Leprology 2014;80(3):229‐34. [DOI: 10.4103/0378-6323.132250; PUBMED: 24823400] - DOI - PubMed
Johansson 2004
-
- Johansson SG, Bieber T, Dahl R, Friedmann PS, Lanier BQ, Lockey RF, et al. Revised nomenclature for allergy for global use: report of the Nomenclature Review Committee of the World Allergy Organization, October 2003. Journal of Allergy and Clinical Immunology 2004;113(5):832‐6. [PUBMED: 15131563] - PubMed
Koch 2014
Langan 2017
-
- Langan SM, Stuart B, Bradshaw L, Schmitt J, Williams HC, Thomas KS. Measuring long‐term disease control in patients with atopic dermatitis: a validation study of well‐controlled weeks. Journal of Allergy and Clinical Immunology 2017;140(6):1580‐6. [DOI: 10.1016/j.jaci.2017.02.043; PUBMED: 28456619] - DOI - PMC - PubMed
Lee 2014
Lewis‐Jones 1995
-
- Lewis‐Jones MS, Finlay AY. The Children's Dermatology Life Quality Index (CDLQI): initial validation and practical use. British Journal of Dermatology 1995;132(6):942‐9. [PUBMED: 7662573] - PubMed
Lewis‐Jones 2001
-
- Lewis‐Jones MS, Finlay AY, Dykes PJ. The Infants' Dermatitis Quality of Life Index. British Journal of Dermatology 2001;144(1):104‐10. [PUBMED: 11167690] - PubMed
Lin 2007
-
- Lin YT, Wang CT, Chiang BL. Role of bacterial pathogens in atopic dermatitis. Clinical Reviews in Allergy & Immunology 2007;33(3):167‐77. [PUBMED: 18163223] - PubMed
Lopes 2013
-
- Lopes C, Silva D, Delgado L, Correia O, Moreira A. Functional textiles for atopic dermatitis: a systematic review and meta‐analysis. Pediatric Allergy and Immunology 2013;24(6):603‐13. [PUBMED: 23980847] - PubMed
Luoma 1983
-
- Luoma R, Koivikko A, Viander M. Development of asthma, allergic rhinitis and atopic dermatitis by the age of five years. A prospective study of 543 newborns. Allergy 1983;38(5):339‐46. [PUBMED: 6614406] - PubMed
Moka 2015
NICE 2013
-
- National Institute for Health and Care Excellence. NICE support for commissioning for atopic eczema in children. www.nice.org.uk/guidance/qs44/resources/support‐for‐commissioning‐for‐at... (accessed prior to 23 October 2019).
Odhiambo 2009
Ong 2016
-
- Ong PY, Leung DY. Bacterial and viral infections in atopic dermatitis: a comprehensive review. Clinical Reviews in Allergy & Immunology 2016;51(3):329‐37. [PUBMED: 27377298] - PubMed
Ravenscroft 2000
-
- Ravenscroft JC, Layton A, Barnham M. Observations on high levels of fusidic acid resistant Staphylococcus aureus in Harrogate, North Yorkshire, UK. Clinical and Experimental Dermatology 2000;25(4):327‐30. [PUBMED: 10971497] - PubMed
Reed 1999
-
- Reed J, Lyons M, Waghorn D, Wilkinson J. Fusidic acid resistance rates in South Buckinghamshire. British Journal of Dermatology 1999;141(Suppl 55):57.
Reginald 2011
-
- Reginald K, Westritschnig K, Linhart B, Focke‐Tejkl M, Jahn‐Schmid B, Eckl‐Dorna J, et al. Staphylococcus aureus fibronectin‐binding protein specifically binds IgE from patients with atopic dermatitis and requires antigen presentation for cellular immune responses. Journal of Allergy and Clinical Immunology 2011;128(1):82‐91. [10.1016/j.jaci.2011.02.034; PUBMED: 21513970] - PubMed
Schafer 2001
-
- Schafer T, Staudt A, Ring J. German instrument for the assessment of quality of life in skin diseases (DIELH). Internal consistency, reliability, convergent and discriminant validity and responsiveness [Deutsches Instrument zur Erfassung der Lebensqualitat bei Hauterkrankungen (DIELH). Interne Konsistenz, Reliabilitat, konvergente und diskriminante Validitat und Veranderungssensitivitat]. Der Hautarzt; Zeitschrift fur Dermatologie, Venerologie, und Verwandte Gebiete 2001;52(7):624‐8. [PUBMED: 11475643] - PubMed
Schmitt 2007
-
- Schmitt J, Langan S, Williams HC, European Dermato‐Epidemiology Network. What are the best outcome measurements for atopic eczema?‐ a systematic review. Journal of Allergy and Clinical Immunology 2007;120(6):1389‐98. [PUBMED: 17910890] - PubMed
Schmitt 2012
-
- Schmitt J, Spuls P, Boers M, Thomas K, Chalmers J, Roekevisch E, et al. Towards global consensus on outcome measures for atopic eczema research: results of the HOME II meeting. Allergy 2012;67(9):1111‐7. [PUBMED: 22844983] - PubMed
Schmitt 2014
-
- Schmitt J, Spuls PI, Thomas KS, Simpson E, Furue M, Deckert S, et al. The Harmonising Outcome Measures for Eczema (HOME) statement to assess clinical signs of atopic eczema in trials. Journal of Allergy and Clinical Immunology 2014;134(4):800‐7. [PUBMED: 25282560] - PubMed
Schulz 2010
Silverberg 2017
-
- Silverberg JI. Public health burden and epidemiology of atopic dermatitis. Dermatology Clinics 2017;35(3):283‐9. [PUBMED: 28577797] - PubMed
Simpson‐Dent 2000
-
- Simpson‐Dent SL, Hunt SJ, Hardman C, Barrett S, Wakelin SH. Rates of fusidic acid resistance in paediatric dermatology patients ‐ a 6‐year study. British Journal of Dermatology 2000;143(Suppl 57):123‐30.
Spuls 2017
-
- Spuls PI, Gerbens LAA, Simpson E, Apfelbacher CJ, Chalmers JR, Thomas KS, et al. Patient‐Oriented Eczema Measure (POEM), a core instrument to measure symptoms in clinical trials: a Harmonising Outcome Measures for Eczema (HOME) statement. British Journal of Dermatology 2017;176(4):979‐84. [PUBMED: 27858989] - PubMed
St John's Institute of Dermatology 2016
-
- St John's Institute of Dermatology. The use of Milton® baths in dermatology. www.guysandstthomas.nhs.uk/resources/patient‐information/dermatology/mil... (accessed prior to 21 October 2019).
Stalder 1994
-
- Stalder JF, Fleury M, Sourisse M, Rostin M, Pheline F, Litoux P. Local steroid therapy and bacterial skin flora in atopic dermatitis. British Journal of Dermatology 1994;131(4):536‐40. [PUBMED: 7947206] - PubMed
Staughton 1984
-
- Staughton RCM, Byrom NA, Nagvekar NM, Hobbs JR. Thymostimulin therapy of adult atopic eczema: a phase I clinical trial. In: Byrom NA, Hobbs JR editor(s). Thymic Factor Therapy. New York: Raven Press, 1984:415‐24.
Stone 2002
-
- Stone KD. Atopic diseases of childhood. Current Opinion in Pediatrics 2002;14(5):634‐46. [PUBMED: 12352260] - PubMed
Strange 1996
-
- Strange P, Skov L, Lisby S, Nielson PL, Baadsgaard O. Staphylococcal enterotoxin B applied on intact normal and intact atopic skin induces dermatitis. Archives of Dermatology 1996;132(1):27‐33. [PUBMED: 8546480] - PubMed
Syed 2015
Thomsen 2015
Totté 2016
-
- Totté JE, Feltz WT, Hennekam M, Belkum A, Zuuren EJ, Pasmans SG. Prevalence and odds of Staphylococcus aureus carriage in atopic dermatitis: a systematic review and meta‐analysis. British Journal of Dermatology 2016;175(4):687‐95. [PUBMED: 26994362] - PubMed
Van Zuuren 2017
Williams 1990
-
- Williams REA, Gibson AG, Aitchison TC, Lever R, Mackie RM. Assessment of a contact‐plate sampling technique and subsequent quantitative bacterial studies in atopic dermatitis. British Journal of Dermatology 1990;123(4):493‐501. [PUBMED: 2095181] - PubMed
Williams 1992
-
- Williams HC. Is the prevalence of atopic dermatitis increasing?. Clinical and Experimental Dermatology 1992;17(6):385‐91. [PUBMED: 1486704] - PubMed
Williams 1994
-
- Williams HC, Burney PG, Hay RJ, Archer CB, Shipley MJ, Hunter JJ, et al. The UK Working Party's diagnostic criteria for atopic dermatitis. I. Derivation of a minimum set of discriminators for atopic dermatitis.. British Journal of Dermatology 1994;131(3):383‐96. [PUBMED: 7918015] - PubMed
Williams 2008
-
- Williams H, Stewart A, Mutius E, Cookson W, Anderson HR, International Study of Asthma and Allergies in Childhood (ISAAC) Phase One and Three Study Groups. Is eczema really on the increase worldwide?. Journal of Allergy and Clinical Immunology 2008;121(4):947‐54.e15. [PUBMED: 18155278] - PubMed
Wollenberg 2014
Yueh 2014
-
- Yueh MF, Taniguchi K, Chen S, Evans RM, Hammock BD, Karin M, et al. The commonly used antimicrobial additive triclosan is a liver tumor promoter. Proceedings of the National Academy of Sciences of the United States of America 2014;111(48):17200‐5. [DOI: 10.1073/pnas.1419119111; PUBMED: 25404284] - DOI - PMC - PubMed
References to other published versions of this review
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

