Bacillus subtilis TerC Family Proteins Help Prevent Manganese Intoxication
- PMID: 31685536
- PMCID: PMC6941523
- DOI: 10.1128/JB.00624-19
Bacillus subtilis TerC Family Proteins Help Prevent Manganese Intoxication
Abstract
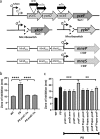
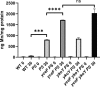
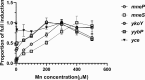
Manganese (Mn) is an essential element and is required for the virulence of many pathogens. In Bacillus subtilis, Mn(II) homeostasis is regulated by MntR, a Mn(II)-responsive, DNA-binding protein. MntR serves as both a repressor of Mn(II) uptake transporters and as a transcriptional activator for expression of two cation diffusion facilitator Mn(II) efflux pumps, MneP and MneS. Mutants lacking either mntR or both mneP and mneS are extremely sensitive to Mn(II) intoxication. Using transposon mutagenesis to select suppressors of Mn(II) sensitivity, we identified YceF, a TerC family membrane protein, as capable of providing Mn(II) resistance. Another TerC paralog, YkoY, is regulated by a Mn(II)-sensing riboswitch and is partially redundant in function with YceF. YkoY is regulated in parallel with an unknown function protein YybP, also controlled by a Mn(II)-sensing riboswitch. Strains lacking between one and five of these known or putative Mn(II) tolerance proteins (MneP, MneS, YceF, YkoY, and YybP) were tested for sensitivity to Mn(II) in growth assays and for accumulation of Mn(II) using inductively coupled plasma mass spectrometry. Loss of YceF and, to a lesser extent, YkoY, sensitizes cells lacking the MneP and MneS efflux transporters to Mn(II) intoxication. This sensitivity correlates with elevated intracellular Mn(II), consistent with the suggestion that TerC proteins function in Mn(II) efflux.IMPORTANCE Manganese homeostasis is primarily regulated at the level of transport. Bacillus subtilis MntR serves as a Mn(II)-activated repressor of importer genes (mntH and mntABC) and an activator of efflux genes (mneP and mneS). Elevated intracellular Mn(II) also binds to Mn-sensing riboswitches to activate transcription of yybP and ykoY, which encodes a TerC family member. Here, we demonstrate that two TerC family proteins, YceF and YkoY, help prevent Mn(II) intoxication. TerC family proteins are widespread in bacteria and may influence host-pathogen interactions, but their effects on Mn(II) homeostasis are unclear. Our results suggest that TerC proteins work by Mn(II) export under Mn(II) overload conditions to help alleviate toxicity.
Keywords: Bacillus subtilis; TerC family; manganese; metal resistance; metalloregulation; regulation; riboswitch.
Copyright © 2020 American Society for Microbiology.
Figures




Similar articles
-
Bacterial manganese sensing and homeostasis.Curr Opin Chem Biol. 2020 Apr;55:96-102. doi: 10.1016/j.cbpa.2020.01.003. Epub 2020 Feb 18. Curr Opin Chem Biol. 2020. PMID: 32086169 Free PMC article. Review.
-
Bacillus subtilis MntR coordinates the transcriptional regulation of manganese uptake and efflux systems.Mol Microbiol. 2017 Jan;103(2):253-268. doi: 10.1111/mmi.13554. Epub 2016 Nov 2. Mol Microbiol. 2017. PMID: 27748968 Free PMC article.
-
Iron homeostasis in Bacillus subtilis relies on three differentially expressed efflux systems.Microbiology (Reading). 2023 Jan;169(1):001289. doi: 10.1099/mic.0.001289. Microbiology (Reading). 2023. PMID: 36748638 Free PMC article.
-
Dysregulation of Magnesium Transport Protects Bacillus subtilis against Manganese and Cobalt Intoxication.J Bacteriol. 2020 Mar 11;202(7):e00711-19. doi: 10.1128/JB.00711-19. Print 2020 Mar 11. J Bacteriol. 2020. PMID: 31964700 Free PMC article.
-
Specificity of metal sensing: iron and manganese homeostasis in Bacillus subtilis.J Biol Chem. 2014 Oct 10;289(41):28112-20. doi: 10.1074/jbc.R114.587071. Epub 2014 Aug 26. J Biol Chem. 2014. PMID: 25160631 Free PMC article. Review.
Cited by
-
Bacterial manganese sensing and homeostasis.Curr Opin Chem Biol. 2020 Apr;55:96-102. doi: 10.1016/j.cbpa.2020.01.003. Epub 2020 Feb 18. Curr Opin Chem Biol. 2020. PMID: 32086169 Free PMC article. Review.
-
The TerC family metal chaperone MeeY enables surfactin export in Bacillus subtilis.J Bacteriol. 2025 May 22;207(5):e0008825. doi: 10.1128/jb.00088-25. Epub 2025 Apr 16. J Bacteriol. 2025. PMID: 40237484 Free PMC article.
-
A Central Role for Magnesium Homeostasis during Adaptation to Osmotic Stress.mBio. 2022 Feb 22;13(1):e0009222. doi: 10.1128/mbio.00092-22. Epub 2022 Feb 15. mBio. 2022. PMID: 35164567 Free PMC article.
-
Why is manganese so valuable to bacterial pathogens?Front Cell Infect Microbiol. 2023 Feb 3;13:943390. doi: 10.3389/fcimb.2023.943390. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 36816586 Free PMC article. Review.
-
Unraveling the Underlying Heavy Metal Detoxification Mechanisms of Bacillus Species.Microorganisms. 2021 Jul 30;9(8):1628. doi: 10.3390/microorganisms9081628. Microorganisms. 2021. PMID: 34442707 Free PMC article. Review.
References
-
- Radin JN, Kelliher JL, Solorzano PKP, Grim KP, Ramezanifard R, Slauch JM, Kehl-Fie TE. 2019. Metal-independent variants of phosphoglycerate mutase promote resistance to nutritional immunity and retention of glycolysis during infection. PLoS Pathog 15:e1007971. doi:10.1371/journal.ppat.1007971. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

