Hsp110 mitigates α-synuclein pathology in vivo
- PMID: 31685606
- PMCID: PMC6883785
- DOI: 10.1073/pnas.1903268116
Hsp110 mitigates α-synuclein pathology in vivo
Abstract
Parkinson's disease is characterized by the aggregation of the presynaptic protein α-synuclein and its deposition into pathologic Lewy bodies. While extensive research has been carried out on mediators of α-synuclein aggregation, molecular facilitators of α-synuclein disaggregation are still generally unknown. We investigated the role of molecular chaperones in both preventing and disaggregating α-synuclein oligomers and fibrils, with a focus on the mammalian disaggregase complex. Here, we show that overexpression of the chaperone Hsp110 is sufficient to reduce α-synuclein aggregation in a mammalian cell culture model. Additionally, we demonstrate that Hsp110 effectively mitigates α-synuclein pathology in vivo through the characterization of transgenic Hsp110 and double-transgenic α-synuclein/Hsp110 mouse models. Unbiased analysis of the synaptic proteome of these mice revealed that overexpression of Hsp110 can override the protein changes driven by the α-synuclein transgene. Furthermore, overexpression of Hsp110 is sufficient to prevent endogenous α-synuclein templating and spread following injection of aggregated α-synuclein seeds into brain, supporting a role for Hsp110 in the prevention and/or disaggregation of α-synuclein pathology.
Keywords: Lewy body; chaperone; disaggregase; proteomics; synapse.
Conflict of interest statement
The authors declare no competing interest.
Figures

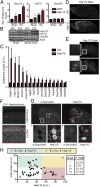

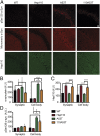

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

