Immunological ignorance is an enabling feature of the oligo-clonal T cell response to melanoma neoantigens
- PMID: 31685621
- PMCID: PMC6876217
- DOI: 10.1073/pnas.1906026116
Immunological ignorance is an enabling feature of the oligo-clonal T cell response to melanoma neoantigens
Abstract
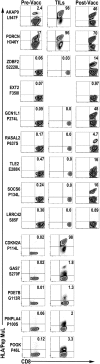
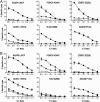
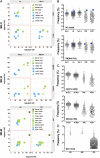
The impact of intratumoral heterogeneity (ITH) and the resultant neoantigen landscape on T cell immunity are poorly understood. ITH is a widely recognized feature of solid tumors and poses distinct challenges related to the development of effective therapeutic strategies, including cancer neoantigen vaccines. Here, we performed deep targeted DNA sequencing of multiple metastases from melanoma patients and observed ubiquitous sharing of clonal and subclonal single nucleotide variants (SNVs) encoding putative HLA class I-restricted neoantigen epitopes. However, spontaneous antitumor CD8+ T cell immunity in peripheral blood and tumors was restricted to a few clonal neoantigens featuring an oligo-/monoclonal T cell-receptor (TCR) repertoire. Moreover, in various tumors of the 4 patients examined, no neoantigen-specific TCR clonotypes were identified despite clonal neoantigen expression. Mature dendritic cell (mDC) vaccination with tumor-encoded amino acid-substituted (AAS) peptides revealed diverse neoantigen-specific CD8+ T responses, each composed of multiple TCR clonotypes. Isolation of T cell clones by limiting dilution from tumor-infiltrating lymphocytes (TILs) permitted functional validation regarding neoantigen specificity. Gene transfer of TCRαβ heterodimers specific for clonal neoantigens confirmed correct TCR clonotype assignments based on high-throughput TCRBV CDR3 sequencing. Our findings implicate immunological ignorance of clonal neoantigens as the basis for ineffective T cell immunity to melanoma and support the concept that therapeutic vaccination, as an adjunct to checkpoint inhibitor treatment, is required to increase the breadth and diversity of neoantigen-specific CD8+ T cells.
Keywords: CD8+ T cells; cancer vaccine; dendritic cells; melanoma; neoantigen.
Conflict of interest statement
The authors declare no competing interest.
Figures


Similar articles
-
Cancer immunotherapy. A dendritic cell vaccine increases the breadth and diversity of melanoma neoantigen-specific T cells.Science. 2015 May 15;348(6236):803-8. doi: 10.1126/science.aaa3828. Epub 2015 Apr 2. Science. 2015. PMID: 25837513 Free PMC article. Clinical Trial.
-
Isolation of T-Cell Receptors Specifically Reactive with Mutated Tumor-Associated Antigens from Tumor-Infiltrating Lymphocytes Based on CD137 Expression.Clin Cancer Res. 2017 May 15;23(10):2491-2505. doi: 10.1158/1078-0432.CCR-16-2680. Epub 2016 Nov 8. Clin Cancer Res. 2017. PMID: 27827318 Free PMC article.
-
Prospective identification of neoantigen-specific lymphocytes in the peripheral blood of melanoma patients.Nat Med. 2016 Apr;22(4):433-8. doi: 10.1038/nm.4051. Epub 2016 Feb 22. Nat Med. 2016. PMID: 26901407 Free PMC article.
-
MHC class II restricted neoantigen: A promising target in tumor immunotherapy.Cancer Lett. 2017 Apr 28;392:17-25. doi: 10.1016/j.canlet.2016.12.039. Epub 2017 Jan 16. Cancer Lett. 2017. PMID: 28104443 Review.
-
Personal Neoantigen Cancer Vaccines: A Road Not Fully Paved.Cancer Immunol Res. 2020 Dec;8(12):1465-1469. doi: 10.1158/2326-6066.CIR-20-0526. Cancer Immunol Res. 2020. PMID: 33262163 Free PMC article. Review.
Cited by
-
Intratumoural administration and tumour tissue targeting of cancer immunotherapies.Nat Rev Clin Oncol. 2021 Sep;18(9):558-576. doi: 10.1038/s41571-021-00507-y. Epub 2021 May 18. Nat Rev Clin Oncol. 2021. PMID: 34006998 Free PMC article. Review.
-
Mixed Response to Cancer Immunotherapy is Driven by Intratumor Heterogeneity and Differential Interlesion Immune Infiltration.Cancer Res Commun. 2022 Jul 28;2(7):739-753. doi: 10.1158/2767-9764.CRC-22-0050. eCollection 2022 Jul. Cancer Res Commun. 2022. PMID: 36923281 Free PMC article.
-
Biochemical and functional characterization of mutant KRAS epitopes validates this oncoprotein for immunological targeting.Nat Commun. 2021 Jul 16;12(1):4365. doi: 10.1038/s41467-021-24562-2. Nat Commun. 2021. PMID: 34272369 Free PMC article.
-
Large-scale transcript variants dictate neoepitopes for cancer immunotherapy.Sci Adv. 2025 Jan 31;11(5):eado5600. doi: 10.1126/sciadv.ado5600. Epub 2025 Jan 31. Sci Adv. 2025. PMID: 39888994 Free PMC article.
-
Expression of inducible factors reprograms CAR-T cells for enhanced function and safety.Cancer Cell. 2022 Dec 12;40(12):1470-1487.e7. doi: 10.1016/j.ccell.2022.11.006. Cancer Cell. 2022. PMID: 36513049 Free PMC article.
References
-
- Coulie P. G., Van den Eynde B. J., van der Bruggen P., Boon T., Tumour antigens recognized by T lymphocytes: At the core of cancer immunotherapy. Nat. Rev. Cancer 14, 135–146 (2014). - PubMed
-
- Schumacher T. N., Scheper W., Kvistborg P., Cancer neoantigens. Annu. Rev. Immunol. 37, 173–200 (2019). - PubMed
-
- Ochsenbein A. F., Immunological ignorance of solid tumors. Springer Semin. Immunopathol. 27, 19–35 (2005). - PubMed
-
- Chen L., Immunological ignorance of silent antigens as an explanation of tumor evasion. Immunol. Today 19, 27–30 (1998). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

