Maternal diabetes induces autism-like behavior by hyperglycemia-mediated persistent oxidative stress and suppression of superoxide dismutase 2
- PMID: 31685635
- PMCID: PMC6876200
- DOI: 10.1073/pnas.1912625116
Maternal diabetes induces autism-like behavior by hyperglycemia-mediated persistent oxidative stress and suppression of superoxide dismutase 2
Abstract
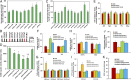
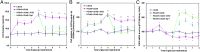
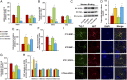
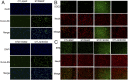
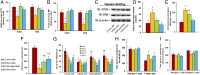
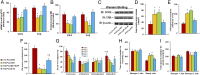
Epidemiological studies show that maternal diabetes is associated with an increased risk of autism spectrum disorders (ASDs), although the detailed mechanisms remain unclear. The present study aims to investigate the potential effect of maternal diabetes on autism-like behavior in offspring. The results of in vitro study showed that transient hyperglycemia induces persistent reactive oxygen species (ROS) generation with suppressed superoxide dismutase 2 (SOD2) expression. Additionally, we found that SOD2 suppression is due to oxidative stress-mediated histone methylation and the subsequent dissociation of early growth response 1 (Egr1) on the SOD2 promoter. Furthermore, in vivo rat experiments showed that maternal diabetes induces SOD2 suppression in the amygdala, resulting in autism-like behavior in offspring. SOD2 overexpression restores, while SOD2 knockdown mimics, this effect, indicating that oxidative stress and SOD2 expression play important roles in maternal diabetes-induced autism-like behavior in offspring, while prenatal and postnatal treatment using antioxidants permeable to the blood-brain barrier partly ameliorated this effect. We conclude that maternal diabetes induces autism-like behavior through hyperglycemia-mediated persistent oxidative stress and SOD2 suppression. Here we report a potential mechanism for maternal diabetes-induced ASD.
Keywords: SOD2; autism spectrum disorders; epigenetics; maternal diabetes; oxidative stress.
Copyright © 2019 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no competing interest.
Figures


References
-
- Langguth M., Fassin M., Alexander S., Turner K. M., Burne T. H. J., No effect of prenatal vitamin D deficiency on autism-relevant behaviours in multiple inbred strains of mice. Behav. Brain Res. 348, 42–52 (2018). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

