Long-Term Exposure to PFE-360 in the AAV-α-Synuclein Rat Model: Findings and Implications
- PMID: 31685675
- PMCID: PMC6978918
- DOI: 10.1523/ENEURO.0453-18.2019
Long-Term Exposure to PFE-360 in the AAV-α-Synuclein Rat Model: Findings and Implications
Abstract
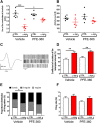
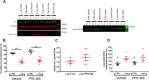
Parkinson's disease (PD) is a progressive neurodegenerative disorder associated with impaired motor function and several non-motor symptoms, with no available disease modifying treatment. Intracellular accumulation of pathological α-synuclein inclusions is a hallmark of idiopathic PD, whereas, dominant mutations in leucine-rich repeat kinase 2 (LRRK2) are associated with familial PD that is clinically indistinguishable from idiopathic PD. Recent evidence supports the hypothesis that an increase in LRRK2 kinase activity is associated with the development of not only familial LRRK2 PD, but also idiopathic PD. Previous reports have shown preclinical effects of LRRK2 modulation on α-synuclein-induced neuropathology. Increased subthalamic nucleus (STN) burst firing in preclinical neurotoxin models and PD patients is hypothesized to be causally involved in the development of the motor deficit in PD. To study a potential pathophysiological relationship between α-synuclein pathology and LRRK2 kinase activity in PD, we investigated the effect of chronic LRRK2 inhibition in an AAV-α-synuclein overexpression rat model. In this study, we report that chronic LRRK2 inhibition using PFE-360 only induced a marginal effect on motor function. In addition, the aberrant STN burst firing and associated neurodegenerative processes induced by α-synuclein overexpression model remained unaffected by chronic LRRK2 inhibition. Our findings do not strongly support LRRK2 inhibition for the treatment of PD. Therefore, the reported beneficial effects of LRRK2 inhibition in similar α-synuclein overexpression rodent models must be considered with prudence and additional studies are warranted in alternative α-synuclein-based models.
Keywords: AAV-α-synuclein; LRRK2; PFE-360; Parkinson’s disease; subthalamic nucleus; α-synuclein.
Copyright © 2019 Andersen et al.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
