The paracrine induction of prostate cancer progression by caveolin-1
- PMID: 31685812
- PMCID: PMC6828728
- DOI: 10.1038/s41419-019-2066-3
The paracrine induction of prostate cancer progression by caveolin-1
Abstract
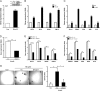
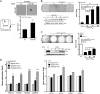
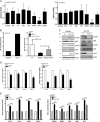
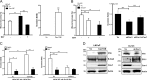
A subpopulation of cancer stem cells (CSCs) plays a critical role of cancer progression, recurrence, and therapeutic resistance. Many studies have indicated that castration-resistant prostate cancer (CRPC) is associated with stem cell phenotypes, which could further promote neuroendocrine transdifferentiation. Although only a small subset of genetically pre-programmed cells in each organ has stem cell capability, CSCs appear to be inducible among a heterogeneous cancer cell population. However, the inductive mechanism(s) leading to the emergence of these CSCs are not fully understood in CRPC. Tumor cells actively produce, release, and utilize exosomes to promote cancer development and metastasis, cancer immune evasion as well as chemotherapeutic resistance; the impact of tumor-derived exosomes (TDE) and its cargo on prostate cancer (PCa) development is still unclear. In this study, we demonstrate that the presence of Cav-1 in TDE acts as a potent driver to induce CSC phenotypes and epithelial-mesenchymal transition in PCa undergoing neuroendocrine differentiation through NFκB signaling pathway. Furthermore, Cav-1 in mCRPC-derived exosomes is capable of inducing radio- and chemo-resistance in recipient cells. Collectively, these data support Cav-1 as a critical driver for mCRPC progression.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



Similar articles
-
Induction of neuroendocrine differentiation in castration resistant prostate cancer cells by adipocyte differentiation-related protein (ADRP) delivered by exosomes.Cancer Lett. 2017 Apr 10;391:74-82. doi: 10.1016/j.canlet.2017.01.018. Epub 2017 Jan 18. Cancer Lett. 2017. PMID: 28109910
-
Dependence of castration-resistant prostate cancer (CRPC) stem cells on CRPC-associated fibroblasts.J Cell Physiol. 2014 Sep;229(9):1170-6. doi: 10.1002/jcp.24546. J Cell Physiol. 2014. PMID: 24752784 Free PMC article.
-
CXCL12γ Promotes Metastatic Castration-Resistant Prostate Cancer by Inducing Cancer Stem Cell and Neuroendocrine Phenotypes.Cancer Res. 2018 Apr 15;78(8):2026-2039. doi: 10.1158/0008-5472.CAN-17-2332. Epub 2018 Feb 5. Cancer Res. 2018. PMID: 29431639 Free PMC article.
-
Regulation of tumor cell plasticity by the androgen receptor in prostate cancer.Endocr Relat Cancer. 2015 Jun;22(3):R165-82. doi: 10.1530/ERC-15-0137. Epub 2015 May 1. Endocr Relat Cancer. 2015. PMID: 25934687 Review.
-
The regulatory pathways leading to stem-like cells underlie prostate cancer progression.Asian J Androl. 2019 May-Jun;21(3):233-240. doi: 10.4103/aja.aja_72_18. Asian J Androl. 2019. PMID: 30178777 Free PMC article. Review.
Cited by
-
Exosomal long noncoding RNA HOXD-AS1 promotes prostate cancer metastasis via miR-361-5p/FOXM1 axis.Cell Death Dis. 2021 Dec 4;12(12):1129. doi: 10.1038/s41419-021-04421-0. Cell Death Dis. 2021. PMID: 34864822 Free PMC article.
-
Extracellular vesicles in anti-tumor drug resistance: Mechanisms and therapeutic prospects.J Pharm Anal. 2024 Jul;14(7):100920. doi: 10.1016/j.jpha.2023.12.010. Epub 2023 Dec 16. J Pharm Anal. 2024. PMID: 39104866 Free PMC article. Review.
-
An inverted CAV1 (caveolin 1) topology defines novel autophagy-dependent exosome secretion from prostate cancer cells.Autophagy. 2021 Sep;17(9):2200-2216. doi: 10.1080/15548627.2020.1820787. Epub 2020 Sep 20. Autophagy. 2021. PMID: 32897127 Free PMC article.
-
Role of caveolin-1 as a biomarker for radiation resistance and tumor aggression in lung cancer.PLoS One. 2021 Nov 11;16(11):e0258951. doi: 10.1371/journal.pone.0258951. eCollection 2021. PLoS One. 2021. PMID: 34762666 Free PMC article.
-
Caveolin-1: an ambiguous entity in breast cancer.Mol Cancer. 2025 Apr 7;24(1):109. doi: 10.1186/s12943-025-02297-8. Mol Cancer. 2025. PMID: 40197489 Free PMC article. Review.
References
-
- Miller Kimberly D., Siegel Rebecca L., Lin Chun Chieh, Mariotto Angela B., Kramer Joan L., Rowland Julia H., Stein Kevin D., Alteri Rick, Jemal Ahmedin. Cancer treatment and survivorship statistics, 2016. CA: A Cancer Journal for Clinicians. 2016;66(4):271–289. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

