Resistance to autosomal dominant Alzheimer's disease in an APOE3 Christchurch homozygote: a case report
- PMID: 31686034
- PMCID: PMC6898984
- DOI: 10.1038/s41591-019-0611-3
Resistance to autosomal dominant Alzheimer's disease in an APOE3 Christchurch homozygote: a case report
Abstract
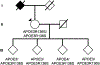
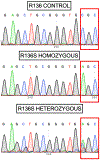
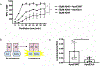
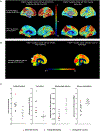
We identified a PSEN1 (presenilin 1) mutation carrier from the world's largest autosomal dominant Alzheimer's disease kindred, who did not develop mild cognitive impairment until her seventies, three decades after the expected age of clinical onset. The individual had two copies of the APOE3 Christchurch (R136S) mutation, unusually high brain amyloid levels and limited tau and neurodegenerative measurements. Our findings have implications for the role of APOE in the pathogenesis, treatment and prevention of Alzheimer's disease.
Conflict of interest statement
Competing interests
P.N.T. has received personal compensation for consulting, serving on scientific advisory boards, or other activities with AbbVie, AC Immune, Acadia, Auspex, Boehringer Ingelheim, Chase Pharmaceuticals, Corium, Eisai, GliaCure, INSYS Therapeutics, Pfizer, T3D, AstraZeneca, Avanir, Biogen, Eli Lilly, H. Lundbeck A/S, Merck and Company, and Roche; holds a provisional patent on “Biomarkers of Alzheimer’s Disease” at the University of Rochester; holds stock options in Adamas; received research support from AstraZeneca, Avanir, Biogen, Eli Lilly, H. Lundbeck A/S, Merck and Company, Roche, Amgen, Avid, Functional Neuromodulation, GE Healthcare, Genentech, Novartis, Takeda, Targacept, the National Institute on Aging, and the Arizona Department of Health Services. H.Z. has served at scientific advisory boards for Roche Diagnostics, Wave, Samumed and CogRx, has given lectures in symposia sponsored by Biogen and Alzecure, and is a co-founder of Brain Biomarker Solutions in Gothenburg AB, a GU Ventures-based platform company at the University of Gothenburg (outside submitted work). R.A.S. has received personal compensation from AC Immune, Eisai, Roche, and Takeda, and research grant support from Eli Lilly and Janssen. All other authors declare no competing financial interests.
Figures
References
-
- Llado A, et al. A novel PSEN1 mutation (K239N) associated with Alzheimer’s disease with wide range age of onset and slow progression. Eur J Neurol 17, 994–996 (2010). - PubMed
-
- Knight WD, et al. Pure progressive amnesia and the APPV717G mutation. Alzheimer Dis Assoc Disord 23, 410–414 (2009). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

