Helix N-Cap Residues Drive the Acid Unfolding That Is Essential in the Action of the Toxin Colicin A
- PMID: 31686499
- PMCID: PMC6899464
- DOI: 10.1021/acs.biochem.9b00705
Helix N-Cap Residues Drive the Acid Unfolding That Is Essential in the Action of the Toxin Colicin A
Abstract
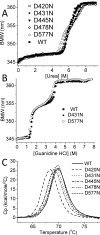
Numerous bacterial toxins and other virulence factors use low pH as a trigger to convert from water-soluble to membrane-inserted states. In the case of colicins, the pore-forming domain of colicin A (ColA-P) has been shown both to undergo a clear acidic unfolding transition and to require acidic lipids in the cytoplasmic membrane, whereas its close homologue colicin N shows neither behavior. Compared to that of ColN-P, the ColA-P primary structure reveals the replacement of several uncharged residues with aspartyl residues, which upon replacement with alanine induce an unfolded state at neutral pH. Here we investigate ColA-P's structural requirement for these critical aspartyl residues that are largely situated at the N-termini of α helices. As previously shown in model peptides, the charged carboxylate side chain can act as a stabilizing helix N-Cap group by interacting with free amide hydrogen bond donors. Because this could explain ColA-P destabilization when the aspartyl residues are protonated or replaced with alanyl residues, we test the hypothesis by inserting asparagine, glutamine, and glutamate residues at these sites. We combine urea (fluorescence and circular dichroism) and thermal (circular dichroism and differential scanning calorimetry) denaturation experiments with 1H-15N heteronuclear single-quantum coherence nuclear magnetic resonance spectroscopy of ColA-P at different pH values to provide a comprehensive description of the unfolding process and confirm the N-Cap hypothesis. Furthermore, we reveal that, in urea, the single domain ColA-P unfolds in two steps; low pH destabilizes the first step and stabilizes the second.
Conflict of interest statement
The authors declare no competing financial interest.
Figures






References
-
- Chenal A.; Prongidi-Fix L.; Perier A.; Aisenbrey C.; Vernier G.; Lambotte S.; Fragneto G.; Bechinger B.; Gillet D.; Forge V.; Ferrand M. (2009) Deciphering Membrane Insertion of the Diphtheria Toxin T Domain by Specular Neutron Reflectometry and Solid-State NMR Spectroscopy. J. Mol. Biol. 391, 872–883. 10.1016/j.jmb.2009.06.061. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

