The relationship between the reporting of euphoria events and early treatment responses to pregabalin: an exploratory post-hoc analysis
- PMID: 31686899
- PMCID: PMC6709807
- DOI: 10.2147/JPR.S199203
The relationship between the reporting of euphoria events and early treatment responses to pregabalin: an exploratory post-hoc analysis
Abstract
Background: Euphoria is a complex, multifactorial problem that is reported as an adverse event in clinical trials of analgesics including pregabalin. The relationship between the reporting of euphoria events and pregabalin early treatment responses was examined in this exploratory post-hoc analysis.
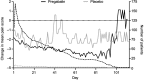
Methods: Data were from patients with neuropathic or non-neuropathic chronic pain enrolled in 40 randomized clinical trials, who received pregabalin (75-600 mg/day) or placebo. Reports of treatment-emergent euphoria events were based on the Medical Dictionary of Regulatory Activities preferred term "euphoric mood". Prevalence rates of euphoria events overall and by indication were assessed. Post-treatment endpoints included ≥30% improvements in pain and sleep scores up to 3 weeks as well as a ≥1-point improvement in daily pain score up to 11 days after treatment.
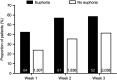
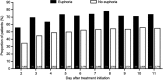
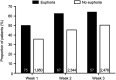
Results: 13,252 patients were analyzed; 8,501 (64.1%) and 4,751 (35.9%) received pregabalin and placebo, respectively. Overall, 1.7% (n=222) of patients reported euphoria events. Among pregabalin-treated patients, a larger proportion who reported euphoria events achieved an early pain response compared with those who did not report euphoria (30% pain responders in week 1 with euphoria events [43.0%], without euphoria events [24.2%]). Results were similar for weeks 2 and 3. For Days 2-11, a larger proportion of pregabalin-treated patients with (relative to without) euphoria events were 1-point pain responders. Findings were similar in pregabalin-treated patients for sleep endpoints (30% sleep responders in week 1 with euphoria events [50.7%], without euphoria events [36.1%]). Similar results were found for weeks 2 and 3. Patients who received placebo showed similar patterns, although the overall number of them who reported euphoria events was small (n=13).
Conclusion: In patients who received pregabalin for neuropathic or non-neuropathic chronic pain, those who experienced euphoria events may have better early treatment responses than those who did not report euphoria events.
Keywords: euphoria; pain; pregabalin; sleep.
© 2019 Parsons et al.
Conflict of interest statement
RF reports consultancy and speaker fees in the past 2 years from AOP Orphan Pharmaceuticals, Eli Lilly, Grünenthal, Merck Selbstmedikation, Mitsubishi Tanabe Pharma, Pfizer Inc., and Scilex Pharmaceuticals, outside of the submitted work. SS is an employee of the University of Western Australia (UWA); within the last 2 years his employer has received honoraria, consulting fees and travel support from Andros Pharmaceuticals, Aspen, bioCSL, Eli Lilly, Grünenthal, Invidior, Janssen, Luye Pharma, Mundipharma, Pfizer Inc., Pierre Fabre, Seqirus, and iX Biopharma. BP, EW, MO, PBB, and LK are employees of Pfizer and have stock or stock options with Pfizer. The authors report no other conflicts of interest in this work.
Figures
References
LinkOut - more resources
Full Text Sources

