Coenzyme Q10
- PMID: 31687097
- PMCID: PMC6822644
- DOI: 10.14797/mdcj-15-3-185
Coenzyme Q10
Abstract
Coenzyme Q10 (CoQ10) is among the most widely used dietary and nutritional supplements on the market. CoQ10 has several fundamental properties that may be beneficial in several clinical situations. This article reviews the pertinent chemical, metabolic, and physiologic properties of CoQ10 and the scientific data and clinical trials that address its use in two common clinical settings: statin-associated myopathy syndrome (SAMS) and congestive heart failure (CHF). Although clinical trials of CoQ10 in SAMS have conflicting conclusions, the weight of the evidence, as seen in meta-analyses, supports the use of CoQ10 in SAMS overall. In CHF, there is a lack of large-scale randomized clinical trial data regarding the use of statins in patients receiving contemporary treatment. However, one relatively recent randomized clinical trial, Q-SYMBIO, suggests an adjunctive role for CoQ10 in CHF. Recommendations regarding the use of CoQ10 in these clinical situations are presented.
Keywords: CoQ10; coenzyme Q10; congestive heart failure; statin myopathy; statin-associated muscle symptoms; ubiquinol; ubiquinone.
© 2019 Houston Methodist Hospital Houston, Texas.
Conflict of interest statement
Conflict of Interest Disclosure: The author has completed and submitted the Methodist DeBakey Cardiovascular Journal Conflict of Interest Statement and none were reported.
Figures
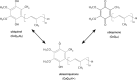

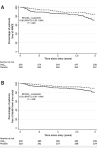
References
-
- Crane FL, Hatefi Y, Lester RL, Widmer C. Isolation of a quinone from beef heart mitochondria. Biochim Biophys Acta. 1957 Jul;25(1):220–1. - PubMed
-
- Bhagavan HN, Chopra RK. Coenzyme Q10: absorption, tissue uptake, metabolism and pharmacokinetics. Free Radic Res. 2006 May;40(5):445–53. - PubMed
-
- Langsjoen PH, Langsjoen AM. Overview of the use of CoQ10 in cardiovascular disease. Biofactors. 1999;9(2–4):273–84. - PubMed
-
- Chopra RK, Goldman R, Sinatra ST, Bhagavan HN. Relative bioavailability of coenzyme Q10 formulations in human subjects. Int J Vitam Nutr Res. 1998;68(2):109–13. - PubMed
-
- Langsjoen PH, Langsjoen AM. Comparison study of plasma Coenzyme Q10 levels in healthy subjects supplemented with ubiquinol versus ubiquinone. Clin Pharmacol Drug Dev. 2014 Jan;3(1):13–7. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

