Gasdermin D Hypermethylation Inhibits Pyroptosis And LPS-Induced IL-1β Release From NK92 Cells
- PMID: 31687364
- PMCID: PMC6800286
- DOI: 10.2147/ITT.S219867
Gasdermin D Hypermethylation Inhibits Pyroptosis And LPS-Induced IL-1β Release From NK92 Cells
Abstract
Introduction: Although natural killer (NK) are major cells used to treat cancer patients, recent clinical trials showed that NK92 cells can be also used for the same purpose due to their high anti-tumor activity. Here, we examined whether these cells might be inflammatory due to the release of interleukin-1β (IL-1β), and whether the anti-inflammatory molecules dimethyl fumarate (DMF), or monomethyl fumarate (MMF) impair this activity.
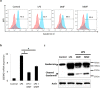
Methods: NK92 cells were examined for the synthesis and release of IL-1β utilizing RT-PCR and ELISA assay, respectively. The expression of hydroxy-carboxylic acid receptors (HCA)1, HCA2 and HCA3 was detected by immunoblotting, flow cytometry, immunofluorescence and RT-PCR assays. The activation of caspase-1 and Gasdermin D (GSDMD) was evaluated by immunoblot assay. Pyroptosis was demonstrated by immunofluorescence imaging. Expression of DNA methyltransferases (DNMTs) mRNA was determined by whole transcriptome and immunoblot analyses.
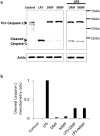
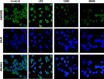
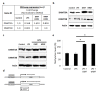
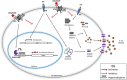
Results: LPS-induced the release of IL-1β from NK92 cells, whereas DMF or MMF inhibited this induction. The effect of these drugs was due to inhibiting the conversion of procaspase-1 into active caspase-1. NK92 cells highly expressed GSDMD, a pyroptotic-mediated molecule. However, LPS induced the distribution of GSDMD into the cell membranes, corroborated with the presence of pyroptotic bodies, an activity that was inhibited by DMF or MMF. These molecule also inhibited the generation of GSDMD through DNMT-mediated hypermethylation of the promoter region of GSDMD gene. These results were supported by increased expression of DNMTs mRNA as determined by whole transcriptome analysis.
Discussion: Our results are the first to show that NK92 cells utilize GSDMD pathway to release IL-1β. Further, DMF and MMF which were previously shown to enhance NK cell cytotoxicity, also inhibit the inflammatory effects of these cells, making them most suitable for treating cancer patients. .
Keywords: IL-1β; NK cells; dimethyl fumarate; gasdermin D; monomethyl fumarate; pyroptosis.
© 2019 Muhammad et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures



References
-
- Rolin J, Sand KL, Knudsen E, Maghazachi AA. FTY720 and SEW2871 reverse the inhibitory effect of S1P on natural killer cell mediated lysis of K562 tumor cells and dendritic cells but not on cytokine release. Cancer Immunol Immunother. 2010;59:575–586. doi: 10.1007/s00262-009-0775-7 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

