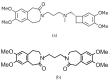
Computational and spectral studies of 3,3'-(propane-1,3-diyl)bis(7,8-dimethoxy-1,3,4,5-tetrahydro-2H-benzo[d]azepin-2-one)
- PMID: 31687545
- PMCID: PMC6819847
- DOI: 10.1016/j.heliyon.2019.e02420
Computational and spectral studies of 3,3'-(propane-1,3-diyl)bis(7,8-dimethoxy-1,3,4,5-tetrahydro-2H-benzo[d]azepin-2-one)
Abstract
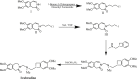
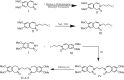
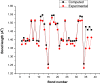
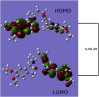
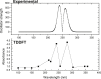
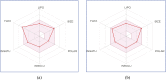
Detection and qualification of unknown impurities during commercial drug synthesis have been mandated by the regulatory authorities. 3,3'-(propane-1,3-diyl)bis(7,8-dimethoxy-1,3,4,5-tetrahydro-2H-benzo [d]azepin-2-one) in short IVA-9, is one such process-related impurity formed during the synthesis of cardiotonic drug Ivabradine. The structure and properties of this molecule have not been explored yet. A suggestive reaction route for the chance formation of IVA-9 during the commercial synthesis of parent drug molecule has been reported in this article. Further, the optimized geometry and vibrational studies have been computed using Gaussian 09. Experimental FTIR scan has also been performed and values show satisfactory consilience with the computational data. The frontier orbital energies and energy band gaps of the reaction fragments and products were computed. The evaluation of ADME parameters such as absorption, distribution, metabolism, and excretion are performed using SwissADME tool to assess the drug-likeness and medicinal chemistry friendliness. Six physiochemical parameters namely flexibility, lipophilicity, size, polarity, solubility and saturation and their critical limits are depicted using the bioavailability radar of the programme to provide insights into pharmacokinetic properties such as human gastrointestinal absorption (HIA), blood-brain-barrier (BBB) permeability, total polar surface area (TPSA) and inhibitor action to important cytochromes etc.
Keywords: ADME studies; Analytical chemistry; Computational; DFT; FTIR; Ivabradine impurity; Pharmaceutical chemistry.
© 2019 Published by Elsevier Ltd.
Figures



References
-
- Satinder A. Marcel Dekker; New York: 1998. Impurities Evaluation of Pharmaceuticals.
-
- Koruth J.S., Lala A., Pinney S., Reddy V.Y., Dukkipati S.R. The clinical use of Ivabradine. J. Am. Coll. Cardiol. 2017;70:1777–1784. - PubMed
-
- Nowakowska J., Pikul P., Marszall M., Ciura K. Application and validation of simple isocratic HPLC-UV-DAD method with dual wavelength detection for Ivabradine determination and its application in the study of stress degradation. J. Chem. 2017 1-7.
-
- Duval D., Hennig P., Bouchet J.P., Vian J., Peglion J.L., Volland J.P., Platzer N., Guilhem J. Stereochemical study of a bradicardisant benzazepine type drug – X-ray structure of the chloride salt and high field NMR study of the stereochemistry in solution. Magn. Reson. Chem. 1997;35:175–183.
LinkOut - more resources
Full Text Sources
Miscellaneous

