Brief freezing steps lead to robust immunofluorescence in the Drosophila nervous system
- PMID: 31687836
- PMCID: PMC7031821
- DOI: 10.2144/btn-2018-0067
Brief freezing steps lead to robust immunofluorescence in the Drosophila nervous system
Abstract
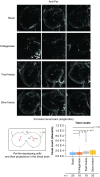
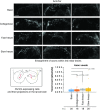
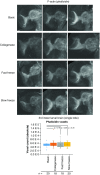
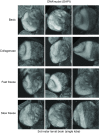
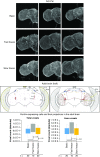
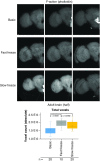
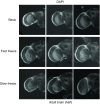
Drosophila melanogaster possesses a complex nervous system, regulating sophisticated behavioral outputs, that serves as a powerful model for dissecting molecular mechanisms underlying neuronal function and neurodegenerative disease. Immunofluorescence techniques provide a way to visualize the spatiotemporal organization of these networks, permitting observation of their development, functional location, remodeling and, eventually, degradation. However, basic immunostaining techniques do not always result in efficient antibody penetration through the brain, and supplemental techniques to enhance permeability can compromise structural integrity, altering spatial organization. Here, slow freezing of brains is shown to facilitate antibody permeability without loss of antibody specificity or brain integrity. To demonstrate the advantages of this freezing technique, the results of two commonly used permeation methods - detergent-based and partial proteolytic digestion - are compared.
Keywords: Drosophila; Drosophila melanogaster; IF; Per; Period; antibody; brain; circadian; flies; fluorescence; freezing; imaging; immunofluorescence; microscopy; nervous system; neural circuit; phalloidin; sleep; staining; wake.
Conflict of interest statement
This research received funding from the University of Missouri Research Board and the NIH Academic Development Via Applied and Cutting Edge Research (ADVANCER) program. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Figures
References
-
- Daul AL, Komori H, Lee CY. Immunofluorescent staining of Drosophila larval brain tissue. Cold Spring Harb. Protoc. 2010(7), pdb prot5460 (2010). - PubMed
-
- Wu JS, Luo L. A protocol for dissecting Drosophila melanogaster brains for live imaging or immunostaining. Nat. Protoc. 1(4), 2110–2115 (2006). - PubMed
-
- Diaper DC, Hirth F. Immunostaining of the developing embryonic and larval Drosophila brain. Methods Mol. Biol. 1082, 3–17 (2014). - PubMed
-
- Kloss B, Price JL, Saez L. et al. The Drosophila clock gene double-time encodes a protein closely related to human casein kinase Iepsilon. Cell 94(1), 97–107 (1998). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
