Resting-State Brain Connectivity Predicts Weight Loss and Cognitive Control of Eating Behavior After Vertical Sleeve Gastrectomy
- PMID: 31689011
- PMCID: PMC6839788
- DOI: 10.1002/oby.22607
Resting-State Brain Connectivity Predicts Weight Loss and Cognitive Control of Eating Behavior After Vertical Sleeve Gastrectomy
Abstract
Objective: The effects of sleeve gastrectomy (SG) on functional connectivity (FC) and associations with weight loss and eating-related cognitive control were investigated.
Methods: In a longitudinal study, 14 SG patients (13 female; 42.1 presurgery BMI) completed study visits 1 month pre surgery and 12 months post surgery. Patients completed the Dutch Eating Behavior Questionnaire and resting-state functional magnetic resonance imaging scanning to measure FC. Data were analyzed using a seed-to-voxel approach in the CONN Toolbox to investigate pre-/postsurgery changes (n = 12) and to conduct predictive analysis (n = 14).
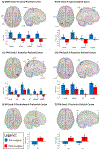
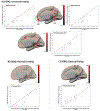
Results: Seed-to-voxel analysis revealed changes in magnitude (decreases) and directionality (positively correlated to anticorrelated) of FC pre to post surgery within and between default mode network, salience network, and frontoparietal network nodes [Family-Wise Error (FWE) corrected at P < 0.05]. Baseline FC of the nucleus accumbens (with insula) and hypothalamus (with precentral gyrus) predicted 12-month post-SG % total weight loss (FWE-P < 0.05). Baseline FC of the hippocampus, frontoparietal network, and default mode network nodes predicted improvement in cognitive control of eating behavior 12 months after SG (FWE-P < 0.05).
Conclusions: Our findings demonstrate changes in FC magnitude and directionality post versus pre surgery within and between resting-state networks and frontal, paralimbic, and visual areas in SG patients. Baseline FC predicted weight loss and changes in cognitive control of food intake behavior at 12 months. These could serve as predictive biomarkers for bariatric surgery.
© 2019 The Obesity Society.
Conflict of interest statement
Disclosure
The authors have no conflicts of interest to disclose. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the National Institutes of Health.
Figures

References
-
- Ness A, Bruce J, Bruce A, Aupperle R, Lepping R, Martin L, et al. Pre-surgical cortical activation to food pictures is associated with weight loss following bariatric surgery. Surg Obes Relat Dis 2014;10: 1188–1195. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

