The gut microbiota is a major regulator of androgen metabolism in intestinal contents
- PMID: 31689143
- PMCID: PMC6962501
- DOI: 10.1152/ajpendo.00338.2019
The gut microbiota is a major regulator of androgen metabolism in intestinal contents
Abstract
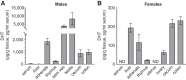
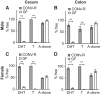
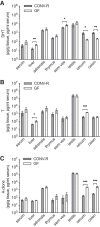
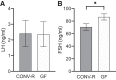
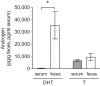
Androgens exert important effects both in androgen-responsive tissues and in the intestinal tract. To determine the impact of the gut microbiota (GM) on intestinal androgen metabolism, we measured unconjugated (free) and glucuronidated androgen levels in intestinal contents from the small intestine, with a low bacterial density, and from cecum and colon, with a high bacterial density. Using a specific, sensitive gas chromatography-tandem mass spectrometry method, we detected high levels of glucuronidated testosterone (T) and dihydrotestosterone (DHT) in small intestinal content of mice of both sexes, whereas in the distal intestine we observed remarkably high levels of free DHT, exceeding serum levels by >20-fold. Similarly, in young adult men high levels of unconjugated DHT, >70-fold higher than in serum, were detected in feces. In contrast to mice with a normal GM composition, germ-free mice had high levels of glucuronidated T and DHT, but very low free DHT levels, in the distal intestine. These findings demonstrate that the GM is involved in intestinal metabolism and deglucuronidation of DHT and T, resulting in extremely high free levels of the most potent androgen, DHT, in the colonic content of young and healthy mice and men.
Keywords: dihydrotestosterone; glucuronidation; gut bacteria; intracrinology; testosterone.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures
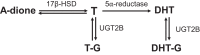

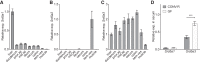
References
-
- Amos-Landgraf JM, Heijmans J, Wielenga MC, Dunkin E, Krentz KJ, Clipson L, Ederveen AG, Groothuis PG, Mosselman S, Muncan V, Hommes DW, Shedlovsky A, Dove WF, van den Brink GR. Sex disparity in colonic adenomagenesis involves promotion by male hormones, not protection by female hormones. Proc Natl Acad Sci USA 111: 16514–16519, 2014. doi:10.1073/pnas.1323064111. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

