Estradiol regulates daily rhythms underlying diet-induced obesity in female mice
- PMID: 31689145
- PMCID: PMC6957379
- DOI: 10.1152/ajpendo.00365.2019
Estradiol regulates daily rhythms underlying diet-induced obesity in female mice
Abstract
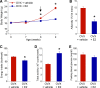
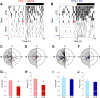
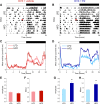
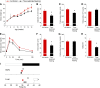
The circadian system is a critical regulator of metabolism and obesity in males, but its role in regulating obesity in females is poorly understood. Because there are sex differences in the development of obesity and susceptibility to obesity-related disorders, we sought to determine the role of estrogens in regulating the circadian mechanisms underlying diet-induced obesity. When fed high-fat diet, C57BL/6J male mice gain weight, whereas females are resistant to diet-induced obesity. Here, we demonstrate that estradiol regulates circadian rhythms in females to confer resistance to diet-induced obesity. We found that ovariectomized females with undetectable circulating estrogens became obese and had disrupted daily rhythms of eating behavior and locomotor activity when fed a high-fat diet. The phase of the liver molecular circadian rhythm was also altered by high-fat diet feeding in ovariectomized mice. Estradiol replacement in ovariectomized females a fed high-fat diet rescued these behavioral and tissue rhythms. Additionally, restoring the daily rhythm of eating behavior in ovariectomized females with time-restricted feeding inhibited diet-induced obesity and insulin resistance. Together, these data suggest that the circadian system is a target for treating obesity and its comorbidities in women after menopause, when circulating levels of estrogens are too low to protect their circadian rhythms.
Keywords: circadian; estrogen; female; ovariectomy; time-restricted feeding.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures

Similar articles
-
High-fat feeding disrupts daily eating behavior rhythms in obesity-prone but not in obesity-resistant male inbred mouse strains.Am J Physiol Regul Integr Comp Physiol. 2021 May 1;320(5):R619-R629. doi: 10.1152/ajpregu.00150.2020. Epub 2021 Feb 24. Am J Physiol Regul Integr Comp Physiol. 2021. PMID: 33626995 Free PMC article.
-
The Estrous Cycle Coordinates the Circadian Rhythm of Eating Behavior in Mice.J Biol Rhythms. 2024 Oct;39(5):413-422. doi: 10.1177/07487304241262356. Epub 2024 Jul 31. J Biol Rhythms. 2024. PMID: 39082411 Free PMC article.
-
High-Fat Feeding Does Not Disrupt Daily Rhythms in Female Mice because of Protection by Ovarian Hormones.Front Endocrinol (Lausanne). 2017 Mar 14;8:44. doi: 10.3389/fendo.2017.00044. eCollection 2017. Front Endocrinol (Lausanne). 2017. PMID: 28352249 Free PMC article.
-
Circadian Rhythms in Diet-Induced Obesity.Adv Exp Med Biol. 2017;960:19-52. doi: 10.1007/978-3-319-48382-5_2. Adv Exp Med Biol. 2017. PMID: 28585194 Review.
-
Response of peripheral rhythms to the timing of food intake.Methods Enzymol. 2015;552:145-61. doi: 10.1016/bs.mie.2014.10.027. Epub 2014 Dec 27. Methods Enzymol. 2015. PMID: 25707276 Review.
Cited by
-
Medial preoptic area FoxO1 controls metabolic adaptation in a sexually dimorphic manner.bioRxiv [Preprint]. 2025 Jun 28:2025.06.25.661575. doi: 10.1101/2025.06.25.661575. bioRxiv. 2025. PMID: 40667056 Free PMC article. Preprint.
-
The Metabolic Syndrome, a Human Disease.Int J Mol Sci. 2024 Feb 13;25(4):2251. doi: 10.3390/ijms25042251. Int J Mol Sci. 2024. PMID: 38396928 Free PMC article. Review.
-
High-fat diet triggers transcriptomic changes in the olfactory bulb.Heliyon. 2025 Jan 22;11(3):e42196. doi: 10.1016/j.heliyon.2025.e42196. eCollection 2025 Feb 15. Heliyon. 2025. PMID: 39927144 Free PMC article.
-
Estrogens and the circadian system.Semin Cell Dev Biol. 2022 Jun;126:56-65. doi: 10.1016/j.semcdb.2021.04.010. Epub 2021 May 9. Semin Cell Dev Biol. 2022. PMID: 33975754 Free PMC article. Review.
-
Maternal high fat diet induces circadian clock-independent endocrine alterations impacting the metabolism of the offspring.iScience. 2024 Jun 21;27(7):110343. doi: 10.1016/j.isci.2024.110343. eCollection 2024 Jul 19. iScience. 2024. PMID: 39045103 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

