Exosomes from mesenchymal stromal cells reduce murine colonic inflammation via a macrophage-dependent mechanism
- PMID: 31689240
- PMCID: PMC6975270
- DOI: 10.1172/jci.insight.131273
Exosomes from mesenchymal stromal cells reduce murine colonic inflammation via a macrophage-dependent mechanism
Abstract
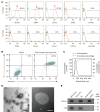
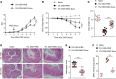
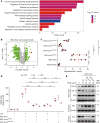
Conventional treatments for inflammatory bowel disease (IBD) have multiple potential side effects. Therefore, alternative treatments are desperately needed. This work demonstrated that systemic administration of exosomes from human bone marrow-derived mesenchymal stromal cells (MSC-Exos) substantially mitigated colitis in various models of IBD. MSC-Exos treatment downregulated inflammatory responses, maintained intestinal barrier integrity, and polarized M2b macrophages but did not favor intestinal fibrosis. Mechanistically, infused MSC-Exos acted mainly on colonic macrophages, and macrophages from colitic colons acquired obvious resistance to inflammatory restimulation when prepared from mice treated with MSC-Exos versus untreated mice. The beneficial effect of MSC-Exos was blocked by macrophage depletion. Also, the induction of IL-10 production from macrophages was partially involved in the beneficial effect of MSC-Exos. MSC-Exos were enriched in proteins involved in regulating multiple biological processes associated with the anticolitic benefit of MSC-Exos. Particularly, metallothionein-2 in MSC-Exos was required for the suppression of inflammatory responses. Taken together, MSC-Exos are critical regulators of inflammatory responses and may be promising candidates for IBD treatment.
Keywords: Inflammatory bowel disease; Stem cell transplantation; Stem cells; Therapeutics.
Conflict of interest statement
Figures





References
-
- Ng SC, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2018;390(10114):2769–2778. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

