Off-the-shelf EBV-specific T cell immunotherapy for rituximab-refractory EBV-associated lymphoma following transplantation
- PMID: 31689242
- PMCID: PMC6994129
- DOI: 10.1172/JCI121127
Off-the-shelf EBV-specific T cell immunotherapy for rituximab-refractory EBV-associated lymphoma following transplantation
Abstract
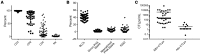
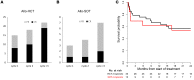
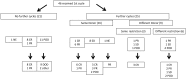
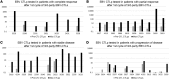
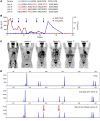
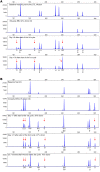
BACKGROUNDAdoptive transfer of donor-derived EBV-specific cytotoxic T-lymphocytes (EBV-CTLs) can eradicate EBV-associated lymphomas (EBV-PTLD) after transplantation of hematopoietic cell (HCT) or solid organ (SOT) but is unavailable for most patients.METHODSWe developed a third-party, allogeneic, off-the-shelf bank of 330 GMP-grade EBV-CTL lines from specifically consented healthy HCT donors. We treated 46 recipients of HCT (n = 33) or SOT (n = 13) with established EBV-PTLD, who had failed rituximab therapy, with third-party EBV-CTLs. Treatment cycles consisted of 3 weekly infusions of EBV-CTLs and 3 weeks of observation.RESULTSEBV-CTLs did not induce significant toxicities. One patient developed grade I skin graft-versus-host disease. Complete remission (CR) or sustained partial remission (PR) was achieved in 68% of HCT recipients and 54% of SOT recipients. For patients who achieved CR/PR or stable disease after cycle 1, one year overall survival was 88.9% and 81.8%, respectively. In addition, 3 of 5 recipients with POD after a first cycle who received EBV-CTLs from a different donor achieved CR or durable PR (60%) and survived longer than 1 year. Maximal responses were achieved after a median of 2 cycles.CONCLUSIONThird-party EBV-CTLs of defined HLA restriction provide safe, immediately accessible treatment for EBV-PTLD. Secondary treatment with EBV-CTLs restricted by a different HLA allele (switch therapy) can also induce remissions if initial EBV-CTLs are ineffective. These results suggest a promising potential therapy for patients with rituximab-refractory EBV-associated lymphoma after transplantation.TRIAL REGISTRATIONPhase II protocols (NCT01498484 and NCT00002663) were approved by the Institutional Review Board at Memorial Sloan Kettering Cancer Center, the FDA, and the National Marrow Donor Program.FUNDINGThis work was supported by NIH grants CA23766 and R21CA162002, the Aubrey Fund, the Claire Tow Foundation, the Major Family Foundation, the Max Cure Foundation, the Richard "Rick" J. Eisemann Pediatric Research Fund, the Banbury Foundation, the Edith Robertson Foundation, and the Larry Smead Foundation. Atara Biotherapeutics licensed the bank of third-party EBV-CTLs from Memorial Sloan Kettering Cancer Center in June 2015.
Keywords: Cancer immunotherapy; Immunology; T cells; Transplantation.
Conflict of interest statement
Figures

Comment in
-
Off-the-shelf EBV-specific T-cell Immunotherapy for EBV-associated PTLD.Transplantation. 2020 Oct;104(10):1972-1973. doi: 10.1097/TP.0000000000003283. Transplantation. 2020. PMID: 32969986 No abstract available.
References
-
- Singavi AK, Harrington AM, Fenske TS. Post-transplant lymphoproliferative disorders. Cancer Treat Res. 2015;165:305–327. - PubMed
-
- San-Juan R, et al. Epstein-Barr virus-related post-transplant lymphoproliferative disorder in solid organ transplant recipients. Clin Microbiol Infect. 2014;20(suppl 7):109–118. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

