Pharmacokinetics and safety of a raltegravir-containing regimen in HIV-infected children aged 2-12 years on rifampicin for tuberculosis
- PMID: 31689263
- PMCID: PMC7232968
- DOI: 10.1097/QAD.0000000000002369
Pharmacokinetics and safety of a raltegravir-containing regimen in HIV-infected children aged 2-12 years on rifampicin for tuberculosis
Abstract
Objectives: Drug-drug interactions limit current antiretroviral treatment options for HIV-infected children with tuberculosis (TB). Rifampicin (RIF) induces UDP-glucuronosyltransferase activity, accelerating the clearance of raltegravir (RAL). We sought to establish an optimal and well tolerated dose of RAL when administered with RIF to HIV and TB co-infected children.
Design: P1101 is a phase I/II open-label dose-finding study of RAL with RIF for children 2 to less than 12 years of age beginning treatment for HIV and active TB.
Setting: Four sites in South Africa.
Methods: Chewable RAL was given at 12 mg/kg per dose twice daily (twice the usual pediatric dose) with two nucleoside reverse transcriptase inhibitors. Intensive RAL pharmacokinetic sampling was conducted 5 to 8 days after antiretroviral therapy was initiated; a fourth antiretroviral agent was then added.
Results: Children were recruited into two age-defined groups: cohort 1 (2 to <6 years old) and cohort 2 (6 to <12 years old). Pharmacological targets [geometric mean (GM) AUC12 h of 14-45 μmol/l h and GM C12 h ≥75 nmol/l) were reached in both cohort 1 (28.8 μmol/l h and 229 nmol/l) and cohort 2 (38.8 μmol/l h and 228 nmol/l). The RAL-based ART was well tolerated by most participants: one participant discontinued treatment because of grade 4 hepatitis that was possibly treatment-related. At week 8, 22 of 24 participants (92%) had HIV RNA concentrations below 400 copies/ml; 19 of 24 (79%) were below 50 copies/ml.
Conclusion: Giving 12 mg/kg twice daily of the chewable RAL formulation achieved pharmacokinetic targets safely in HIV-infected children receiving RIF for TB.
Figures
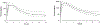
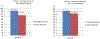
References
-
- World Health Organization. Global tuberculosis report 2018. 2018.
-
- Lawn SD, Myer L, Bekker LG, Wood R. Burden of tuberculosis in an antiretroviral treatment programme in sub-Saharan Africa: impact on treatment outcomes and implications for tuberculosis control. AIDS 2006; 20(12):1605–1612. - PubMed
-
- Fairlie L, Muchiri E, Beylis CN, Meyers T, Moultrie H. Microbiological investigation for tuberculosis among HIV-infected children in Soweto, South Africa. Int J Tuberc Lung Dis 2014; 18(6):676–681. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

